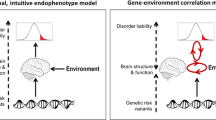
Abstract
Gene–environment interaction research in psychiatry is new, and is a natural ally of neuroscience. Mental disorders have known environmental causes, but there is heterogeneity in the response to each causal factor, which gene–environment findings attribute to genetic differences at the DNA sequence level. Such findings come from epidemiology, an ideal branch of science for showing that gene–environment interactions exist in nature and affect a significant fraction of disease cases. The complementary discipline of epidemiology, experimental neuroscience, fuels gene–environment hypotheses and investigates underlying neural mechanisms. This article discusses opportunities and challenges in the collaboration between psychiatry, epidemiology and neuroscience in studying gene–environment interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Caspi, A. et al. Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854 (2002).
Levinson, D. F. The genetics of depression. Biol. Psychiatry 18 Nov 2005 (doi: 10.1016/j.biopsych.2005.08.024).
Owen, M. J., Williams, N. H. & O'Donovan, M. C. The molecular genetics of schizophrenia: new findings promise new insights. Mol. Psychiatry 9, 14–27 (2004).
Goldman, D., Orozsi, G. & Ducci, F. The genetics of addictions: uncovering the genes. Nature Rev. Genet. 6, 521–532 (2005).
Insel, T. & Collins, F. S. Psychiatry in the genomics era. Am. J. Psychiatry 160, 616–620 (2003).
Hamer, D. Rethinking behavior genetics. Science 298, 71–72 (2002).
Gottesman, I. I. & Gould, T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645 (2003).
Moffitt, T. E., Caspi, A. & Rutter, M. Strategy for investigating interactions between measured genes and measured environments. Arch. Gen. Psychiatry 62, 473–481 (2005).
Moffitt, T. E. The new look of behavioral genetics in developmental psychopathology. Psychol. Bull. 131, 533–554 (2005).
Rutter, M. Environmentally mediated risks for psychopathology: research strategies and findings. J. Am. Acad. Child. Adolesc. Psychiatry 44, 3–18 (2005).
Heath, A. C. & Nelson, E. C. Effects of the interaction between genotype and environment: research into the genetic epidemiology of alcohol dependence. Alcohol Res. Health 26, 193–201 (2002).
Loeber, R. & Farrington, D. P. Serious and Violent Juvenile Offenders: Risk Factors and Successful Interventions (Sage, Thousand Oaks, California, 1998).
Kendler, K. S., Gardner, C. O. & Prescott, C. A. Toward a comprehensive developmental model for major depression in women. Am. J. Psychiatry 159, 1133–1145 (2002).
Tsuang, M. T., Stone, W. S. & Faraone, S. V. Genes, environment and schizophrenia. Br. J. Psychiatry 178, S18–S24 (2001).
van Os, J., Krabbendam, L., Myin-Gerneys, L. & Delespaul, P. The schizophrenia environment. Curr. Opin. Psychiatry 18, 141–145 (2005).
Plomin, R., DeFries, J. C., McClearn, G. E. & McGuffin, P. Behavioral Genetics (W. H. Freeman, New York, 2001).
King, M. -C., Marks, J. H. & Mandell, J. B. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302, 643–646 (2003).
O'Rahilly, S., Barroso, I. & Wareham, N. J. Genetic factors in type 2 diabetes: the end of the beginning? Science 307, 370–373 (2005).
Corella, D. & Ordovas, J. M. Single nucleotide polymorphisms that influence lipid metabolism: interaction with dietry factors. Annu. Rev. Nutr. 25, 341–390 (2005).
Smith, M. W. et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science 277, 959–965 (1997).
Kotb, M. et al. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nature Med. 8, 1398–1404 (2002).
Kleeberger, S. R. & Peden, D. Gene-environment interactions in asthma and other respiratory diseases. Annu. Rev. Med. 56, 383–400 (2005).
Caspi, A. et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–899 (2003).
Caspi, A. et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the COMT gene: longitudinal evidence of a gene X environment interaction. Biol. Psychiatry 57, 1117–1127 (2005).
Brookes, K. -J. et al. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Arch. Gen. Psychiatry 63, 74–81 (2005).
Thapar, A. et al. Catechol-O-methyltransferase gene variant and birth weight predict early-onset antisocial behavior in children with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 62, 1275–1278 (2005).
Koenen, K. C. et al. Polymorphisms in FKBP5 are associated with peri-traumatic dissociation in medically injured children. Mol. Psychiatry 10, 1058–1059 (2005).
Khouri, M. J., Millikan, R., Little, J. & Gwinn, M. The emergence of epidemiology in the genomics age. Int. J. Epidemiol. 33, 936–944 (2004).
Murphy, D. L. et al. Genetic perspectives on the serotonin transporter. Brain. Res. Bull. 56, 487–494 (2001).
Bennett, A. J. et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol. Psychiatry 7, 188–122 (2002).
Hariri, A. R. et al. Serotonin transporter genetic variation and the response of the human amygdala. Science 297, 400–404 (2002).
Moffitt, T. E., Caspi, A. & Rutter, M. Measured gene-environment interactions in psychopathology: concepts, research strategies and implications for research, intervention and public understanding of genetics. Persp. Psychol. Sci. 1, 5–27 (2006).
Hunter, D. J. Gene-environment interactions in human diseases. Nature Rev. Genet. 6, 287–298 (2005).
Foley, D. L. et al. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Arch. Gen. Psychiatry 61, 738–744 (2004).
Haberstick, B. C. et al. Monoamine oxidase A (MAOA) and antisocial behaviors in the presence of childhood and adolescent maltreatment. Am. J. Med. Genet 135B, 59–64 (2005).
Kim-Cohen, J. et al. MAOA, early adversity and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol. Psychiatry (in the press).
Nilsson, K. W. et al. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol. Psychiatry 59, 121–127 (2005).
Young, S. E. et al. Interaction between MAO-A genotype and maltreatment in the risk for conduct disorder: failure to confirm in adolescent patients. Am. J. Psychiatry 163, 1019–1025 (2006).
Eley, T. C. et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol. Psychiatry 9, 908–915 (2004).
Grabe, H. J. et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol. Psychiatry 10, 220–224 (2005).
Jacobs, N. et al. Stress-related negative affectivity and genetically reduced 5-HTT function: evidence for synergism in shaping risk for depression. Arch. Gen. Psychiatry (in the press).
Kaufman, J. et al. Social support and serotonin transporter gene moderate depression in maltreated children. Proc. Natl Acad. Sci. 101, 17316–17321 (2004).
Kaufman, J. et al. BDNF-5HTTLPR gene interactions and environmental modifiers of depression in children. Biol. Psychiatry 59, 673–680 (2006).
Kendler, K. S., Kuhn, J. W., Vittum, J., Prescott, C. A. & Riley, B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch. Gen. Psychiatry 62, 529–535 (2005).
Wilhelm, K. A. et al. Life events, first depression onset and the serotonin transporter gene. Br. J. Psychiatry 188, 210–215 (2006).
Zalsman, G. et al. A triallelic serotonin transporter gene promoter polymorphism. Am. J. Psychiatry (in the press).
Mandelli, L. et al. Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful like events in mood disorders. Int. J. Neuropsychopharmacol. 7 Jun 2006 (doi:10.1017/51461145706006882).
Gillespie, N. A., Whitfield, J. B., Williams, B., Heath, A. C. & Martin, N. G. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol. Med. 35, 101–111 (2005).
Surtees, P. G. et al. Social adversity, the serotonin transporter (5HTTLPR) polymorphism and major depressive disorder. Biol. Psychiatry 59, 224–229 (2006).
Merikangas, K. & Risch, N. Will the genomics revolution revolutionise psychiatry? Am. J. Psychiatry 160, 625–635 (2003).
Ansorge, M. S., Zhou, M., Lira, A., Hen, R. & Gingrich, J. A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306, 879–881 (2004).
Fox, N. et al. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychol. Sci. 16, 921–926 (2005).
Hariri, A. R. et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch. Gen. Psychiatry 62, 146–152 (2005).
Pezawas, L. et al. 5-HTTLPR polymorphism impacts human cingulate-amydala interactions: A genetic susceptibility mechanism for depression. Nature Neurosci. 8, 828–834 (2005).
Cases, O. et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 268, 1763–1766 (1995).
Brunner, H. G., Nelen, M., Breakefield, X. O., Ropers, H. H. & Vanoost, B. A. Abnormal-behavior associated with a point mutation in the structural gene for monoamine oxidase-A. Science 262, 578–580 (1993).
Meyer-Lindenberg, A. et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl Acad. Sci. 103, 6269–6274 (2006).
Bearden, C., Reus, V. I. & Freimer, N. B. Why genetic investigation of psychiatric disorders is so difficult. Curr. Opin. Genet. Dev. 14, 280–286 (2004).
Colhoun, H. M., McKeigue, P. M. & Smith, G. D. Problems of reporting genetic associations with complex outcomes. Lancet 361, 865–872 (2003).
Lohmueller, K. E., Pearce, C. L., Pike, M., Lander, E. S. & Hirschhorn, J. N. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nature Genet. 33, 177–182 (2003).
Plomin, R. & Crabbe, J. DNA. Psychol. Bull. 126, 806–828 (2000).
McClelland, G. H. & Judd, C. M. Statistical difficulties of detecting interactions and moderator effects. Psychol. Bull. 114, 376–390 (1993).
Plomin, R. & Bergeman, C. S. The nature of nurture: genetic influence on 'environmental' measures. Behav. Brain. Sci. 14, 373–386 (1991).
Rutter, M., Moffitt, T. E. & Caspi, A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J. Child. Psychol. Psychiatry 47, 226–261 (2006).
Wong, M. Y., Day, N. E., Luan, J. A., Chan, K. P. & Wareham, N. J. The detection of gene-environment interaction for continuous traits: should we deal with measurment error by bigger studies or better measurement? Int. J. Epidemiol. 32, 51–57 (2003).
Sullivan, P. F., Eaves, L. J., Kendler, K. S. & Neale, M. C. Genetic case-control association studies in neuropsychiatry. Arch. Gen. Psychiatry. 58, 1015–1024 (2001).
Cornelis, M. C., El-Sohemy, A., Kabagambe, E. K. & Campos, H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 10, 1135–1141 (2006).
Cronbach, L. J. & Meehl, P. E. Construct validity in psychological tests. Psychol. Bull. 52, 281–302 (1955).
Hariri, A. R. & Holmes, A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn. Sci. 10, 182–191 (2006).
Leonardo, E. D. & Hen, R. Genetics of affective and anxiety disorders. Annu. Rev. Psychol. 57, 117–137 (2006).
Crabbe, J. C. in Behavioral Genetics in the Postgenomic Era (eds Plomin, R., DeFries, J. C., Craig, I. W. & McGuffin, P.) 291–308 (American Psychological Association, Washington, 2003).
Flint, J. in Behavioral Genetics in the Postgenomic Era (eds Plomin, R., DeFries, J. C., Craig, I. W. & McGuffin, P.) 425–442 (American Psychological Association, Washington DC, 2003).
Cryan, J. & Holmes, A. The ascent of mouse: advances in modelling human depression and anxiety. Nature Rev. Drug Discov. 4, 775–790 (2005).
Carola, V., Frazzetto, G. & Gross, C. Identifying interactions between genes and early environment in the mouse. Genes Brain Behav. 5, 189–199 (2006).
Holmes, A., Murphy, D. L. & Crawley, J. N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol. Psychiatry 54, 953–959 (2003).
Lipoldova, M. & Demant, P. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nature Rev. Genet. 7, 294–305 (2006).
Egan, M. F. et al. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl Acad. Sci. 98, 6917–6922 (2001).
Egan, M. F. et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 (2003).
Hariri, A. R., Drabant, E. M. & Weinberger, D. R. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol. Psychiatry 59, 888–897 (2006).
Heinz, A. et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neurosci. 8, 20–21 (2005).
Fallgatter, A. J. et al. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology 29, 1506–1511 (2004).
Battaglia, M. et al. Influence of the serotonin transporter promoter gene and shyness on children's cerebral responses to facial expressions. Arch. Gen. Psychiatry 62, 85–94 (2005).
Finley, J. C. et al. A genetic polymorphisn of the α-2-adrenergic receptor increases autonomic responses to stress. J. Appl. Physiol. 96, 2231–2239 (2004).
Wust, A. F. et al. Common polymorphisms in the glucocorticoid receptor gene are associated with the adrenocorticol responses to psychosocial stress. J. Clin. Endocrinol. Metab. 89, 565–573 (2004).
Collins, F. S. The case for a US prospective cohort study of genes and environment. Nature 429, 475–477 (2004).
Wright, A. F., Carothers, A. D. & Campbell, H. Gene-environment interactions — the Biobank UK study. J. Pharmacogenomics 2, 75–82 (2002).
Hattori, E., Liu, C., Zhu, H. & Gershon, E. S. Genetic tests of biological systems in affective disorders. Mol. Psychiatry 10, 719–740 (2005).
Charney, D. S. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatry 161, 195–216 (2004).
de Kloet, E. R., Joë ls, M. & Holsboer, F. Stress and the brain: from adaptation to disease. Nature Rev. Neurosci. 6, 463–475 (2005).
Heim, C., Plotsky, P. M. & Nemeroff, C. B. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 29, 641–648 (2004).
Hirschhorn, J. N. & Daly, M. J. Genome-wide association studies for common diseases and complex traits. Nature Rev. Genet. 6, 95–108 (2005).
Rutter, M., Caspi, A. & Moffitt, T. E. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J. Child. Psychol. Psychiatry 44, 1092–1115 (2003).
Arseneault, L., Cannon, M., Witton, J. & Murray, R. M. Causal association between cannabis and psychosis: examination of the evidence. Br. J. Psychiatry 184, 110–117 (2004).
Henquet, C. et al. Prospective cohort study of cannabis use, predisposition for psychosis and psychotic symptoms in young people. Br. Med. J. 330, 11–15 (2005).
Solowij, N. S. et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287, 1123–1131 (2002).
Bunney, W. W. & Bunney, B. G. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res. Rev. 31, 138–146 (2000).
Weinberger, D. R. et al. Prefontal neurons and the genetics of schizophrenia. Biol. Psychiatry 50, 825–844 (2001).
Henquet, C. et al. An experimental study of COMT Val-158-Met moderation of cannabis-induced effects on psychosis and cognition (manuscript in preparation).
Castle, D. & Murray, R. Marijuana and Madness (Cambridge University Press, Cambridge, 2004).
Hutchison, K. E., McGeary, J., Smolen, A., Brayn, A. & Swift, R. M. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health Psychol. 21, 139–149 (2002).
Hutchison, K. E. et al. Olanzapine reduces craving for alcohol: a DRD4 VNTR polymorphism by pharmacotherapy interaction. Neuropsychopharmacology 28, 1882–1888 (2003).
Hutchison, K. E. et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology 31, 1310–1317 (2006).
Hutchison, K. E., La Chance, H., Naiura, R., Bryan, A. & Smolen, A. The DRD4 VNTR polymorphism influences reactivity to smoking cues. J. Abnorm. Psychol. 111, 134–143 (2002).
Slutske, W. S., Caspi, A., Moffitt, T. E. & Poulton, R. Personality and problem gambling: a prospective study of a birth cohort of young adults. Arch. Gen. Psychiatry. 69, 769–775 (2005).
Barr, C. S. et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatry 55, 733–738 (2004).
Acknowledgements
Supported by the UK Medical Research Council, the National Institute of Mental Health, National Institutes of Health, the William T. Grant Foundation, and Royal Society Wolfson Merit Awards to T.E.M. and A.C. We thank the reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Caspi, A., Moffitt, T. Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 7, 583–590 (2006). https://doi.org/10.1038/nrn1925
Issue Date:
DOI: https://doi.org/10.1038/nrn1925
This article is cited by
-
Amygdala subdivisions exhibit aberrant whole-brain functional connectivity in relation to stress intolerance and psychotic symptoms in 22q11.2DS
Translational Psychiatry (2023)
-
Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality
Biology of Sex Differences (2022)
-
Time moderates the interplay between 5-HTTLPR and stress on depression risk: gene x environment interaction as a dynamic process
Translational Psychiatry (2022)