Key Points
-
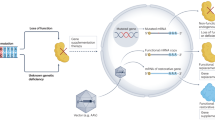
Acute neurological insults disrupt neuronal energy profiles and produce hyperexcitation in glutamatergic synapses. This leads to excessive synaptic glutamate accumulation and pathological accumulation of free cytosolic calcium in postsynaptic terminals. The excess calcium triggers degenerative events, including cytoskeletal degradation, collapse of mitochondrial potentials, protein misfolding, generation of reactive oxygen species (ROS) and oxidative damage.
-
The neuronal damage caused by acute insults could be targeted by gene therapy at several levels, including hyperexcitability, glutamate or calcium excess, ROS accumulation, inflammation and neurotoxicity.
-
From a clinical perspective, it might be argued that it does not matter how a transgene protects neurons, as long as it does. However, understanding the mechanisms that underlie protection will undoubtedly help to optimize the approach.
-
Does the sparing of neurons from death also spare them from dysfunction? These events can sometimes be dissociated; for example, FGF2 overexpression spares retinal photoreceptors from light-induced degeneration, but does not restore their function. In other cases, it is expected that function is more likely to be spared if cell death is halted before metabolic collapse and oxidative damage are allowed to occur.
-
It will be important to target the earliest steps of toxicity following insults. However, the adverse effects of expressing a transgene constitutively pre-insult might outweigh the benefits. An alternative approach would be to introduce an inducible vector pre–insult and induce it at the time of the insult, or to use a vector that is induced by the insult itself.
-
The next stage will be to make the transition from showing that a particular transgene can be neuroprotective, to finding out which intervention is most effective under a particular pathological circumstance.
Abstract
In recent years, there has been tremendous progress in uncovering the molecular, cellular and systemic factors that give rise to neuronal death following acute neurological insults. This has fuelled a burgeoning interest in the use of gene transfer techniques to protect neurons from such insults. This review describes these gene therapy strategies from the perspective of the biology of neuronal death, considering which steps in the cell death cascades have been successfully targeted in pre-clinical studies, and how this research might be applied to the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Costantini, L. C., Bakowska, J. C., Breakefield, X. O. & Isacson, O. Gene therapy in the CNS. Gene Ther. 7, 93–109 (2000).
Fink, D. J., deLuca, N. A., Yamada, M., Wolfe, D. P. & Glorioso, J. C. Design and application of HSV vectors for neuroprotection. Gene Ther. 7, 115–119 (2000).
Hsich G., Sena-Esteves M. & Breakefield X. O. Critical issues in gene therapy for neurologic disease. Hum. Gene Ther. 13, 579–604 (2002). An excellent overview of the broad subject of neuronal gene therapy.
Janson, C. G., McPhee, S. W., Leone, P., Freese A. & During M. J. Viral-based gene transfer to the mammalian CNS for functional genomic studies. Trends Neurosci. 24, 706–712 (2001).
Lee, J. M., Zipfel, G. J. & Choi D. W. The changing landscape of ischaemic brain injury mechanisms. Nature 399 (Suppl.), A7–A14 (1999). A superb and authoritative review of the pathophysiology of neuron death following necrotic insults.
Yuan, J. & Yankner, B. A. Apoptosis in the nervous system. Nature 407, 802–809 (2000).
Roy, M. & Sapolsky, R. Neuronal apoptosis in acute necrotic insults: why is this subject such a mess? Trends Neurosci. 22, 419–422 (1999).
Sperandio, S., de Belle, I. & Bredesen, D. E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl Acad. Sci. USA 97, 14376–14381 (2001). | PubMed
Joza, N., et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410, 549–554 (2001).
Dare, E., Gotz, M. E., Zhivotovsky, B., Manzo, L. & Ceccatelli, S. Antioxidants J811 and 17β-estradiol protect cerebellar granule cells from methylmercury-induced apoptotic cell death. J. Neurosci. Res. 62, 557–565 (2000).
Abele, A. E. & Miller, R. J. Potassium channel activators abolish excitotoxicity in cultured hippocampal pyramidal neurons. Neurosci. Lett. 115, 195–200 (1990).
Lauritzen, I., De Weille J. R. & Lazdunski, M. The potassium channel opener (–)-cromakalim prevents glutamate-induced cell death in hippocampal neurons. J. Neurochem. 69, 1570–1579 (1997).
Lee, A. L. et al. Potassium channel gene therapy can prevent neuron death resulting from necrotic insults. Soc. Neurosci. Abstr. (in the press).
Slimko, E., McKinney, S., Anderson, D., Davidson, N. & Lester H. Selective electrical silencing of mammalian neurons in vitro by the use of intertebrate ligand-gated chloride channels. J. Neurosci. 22, 7373–7379 (2002).
Robert, J. J. et al. Adenovirus-mediated transfer of a functional GAD gene into nerve cells: potential for the treatment of neurological disease. Gene Ther. 4, 1237–1245 (1997).
Filippova, N. et al. Recombinant GABAC receptor expressed in rat hippocampal neurons after infection with an adenovirus containing the human rho1 subunit. J. Physiol. (Lond). 535, 145–153 (2001).
Ho, D. Y., Saydam, T. C., Fink, S. L., Lawrence, M. S. & Sapolsky, R. M. Defective herpes simplex virus vectors expressing the rat brain glucose transporter protect cultured neurons from necrotic insults. J. Neurochem. 65, 842–850 (1995).
Lawrence, M. S., Ho, D. Y., Dash, R. & Sapolsky, R. M. Herpes simplex virus vectors overexpressing the glucose transporter gene protect against seizure-induced neuron loss.. Proc. Natl Acad. Sci. USA 92, 7247–7251 (1995). One of the earliest demonstrations of overexpressing a neuroprotective gene to protect against a necrotic insult.
Lawrence, M. S. et al. Overexpression of the glucose transporter gene with a herpes simplex viral vector protects striatal neurons against stroke. J. Cereb. Blood Flow Metab. 16, 181–186 (1996).
Magistretti, P. J., Pellerin, L., Rothman, D. L. & Shulman, R. G. Energy on demand. Science 283, 496–497 (1999).
Dienel, G. A. & Hertz, L. Glucose and lactate metabolism during brain activation. J. Neurosci. Res. 66, 824–838 (2001).
McIntosh, L. et al. Overexpression of the lactate transporter in neurons or the glucose transporter in glia protects neurons against excitotoxic insults. Soc. Neurosci. Abstr. (in the press).
Kauppinen, R. A., McMahon, H. T. & Nicholls, D. G. Ca2+-dependent and Ca2+-independent glutamate release, energy status and cytosolic free Ca2+ concentration in isolated nerve terminals following metabolic inhibition: possible relevance to hypoglycaemia and anoxia. Neuroscience 27, 175–182 (1988).
Wahlestedt, C. et al. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nature 363, 260–263 (1993). A first demonstration of the use of an antisense gene therapy strategy against a necrotic neurological insult.
McMahon, A. et al. Calbindin-D28k buffers intracellular calcium and promotes resistance to degeneration in PC12 cells. Brain Res. Mol. Brain Res. 54, 56–63 (1998).
Prehn, J. H. et al. Protective effect of transforming growth factor-β1 on β-amyloid neurotoxicity in rat hippocampal neurons. Mol. Pharmacol. 49, 319–328 (1996).
Meier, T. J., Ho, D. Y. & Sapolsky, R. M. Increased expression of calbindin D28k via herpes simplex virus amplicon vector decreases calcium ion mobilization and enhances neuronal survival following hypoglycemic challenge. J. Neurochem. 69, 1039–1047 (1997).
Phillips, R. G. et al. Gene therapy effectiveness differs for neuronal survival and behavioral performance. Gene Ther. 8, 579–585 (2001).
Monje, M. L., Phillips, R. & Sapolsky, R. Calbindin overexpression buffers hippocampal cultures from the energetic impairments caused by glutamate. Brain Res. 911, 37–42 (2001).
Phillips, R. G. et al. Calbindin D28K gene-transfer via herpes simplex virus amplicon vector decreases hippocampal damage in vivo following neurotoxic insults. J. Neurochem. 73, 1200–1205 (1999).
Yenari, M. A. et al. Calbindin D28K overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke 32, 1028–1035 (2001). | Article
Ohtsuki, T., Matsumoto, M., Suzuki, K., Taniguchi, N. & Kamada, T. Effect of transient forebrain ischemia on superoxide dismutases in gerbil hippocampus. Brain Res. 620, 305–309 (1993).
Barkats, M., Millecamps, S., Abrioux, P., Geoffroy, M. C. & Mallet, J. Overexpression of glutathione peroxidase increases resistance of neuronal cells to Aβ-mediated neurotoxicity. J. Neurochem. 75, 1438–1446 (2000).
Brooke, S., Wang, H., Cheng, E., Nimon, T. & Sapolsky, R. Overexpression of antioxidant enzymes protects cultured hippocampal and cortical neurons from acute insults. Soc. Neurosci. Abstr. (in the press).
Patel, R. et al. Disruptive effects of glucocorticoids on glutathione peroxidase biochemistry in hippocampal cultures. J. Neurochem. 82, 118–125 (2002).
Przedborski, S. et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 12, 1658–1667 (1992).
Murakami, K., Kondo, T., Epstein, C. J. & Chan, P. H. Overexpression of CuZn-superoxide dismutase reduces hippocampal injury after global ischemia in transgenic mice. Stroke 28, 1797–1804 (1997). | Article
Fink, S. L., Chang, L. K., Ho, D. Y. & Sapolsky, R. M. Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J. Neurochem. 68, 961–969 (1997).
Amin, V., Cumming, D. V. & Latchman, D. S. Over-expression of heat shock protein 70 protects neuronal cells against both thermal and ischaemic stress but with different efficiencies. Neurosci. Lett. 206, 45–48 (1996).
Yenari, M. A. et al. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann. Neurol. 44, 584–591 (1998).
Hoehn, B. et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J. Cereb. Blood Flow Metab. 21, 1303–1309 (2001).
Wagstaff, M. J. et al. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J. Biol. Chem. 274, 5061–5069 (1999).
Lawrence, M. S., Ho, D. Y., Sun, G. H., Steinberg, G. K. & Sapolsky, R. M. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurologic insults in vitro and in vivo. J. Neurosci. 16, 486–496 (1996).
Jia, W. W. et al. A bcl-2 expressing viral vector protects cortical neurons from excitotoxicity even when administered several hours after the toxic insult. Brain Res. Mol. Brain Res. 42, 350–353 (1996). An early demonstration that gene therapy can be carried out following a necrotic insult, rather than in anticipation of one.
Tamatani, M. et al. A pathway of neuronal apoptosis induced by hypoxia/reoxygenation: roles of nuclear factor -κB and Bcl-2. J. Neurochem. 75, 683–693 (2000).
McLaughlin, J. et al. Sparing of neuronal function post-seizure with gene therapy. Proc. Natl Acad. Sci. USA 97, 12804–12809 (2000). An early demonstration that gene therapy strategies that spare neurons from death do not necessarily spare those neurons from dysfunction.
Phillips, R. G., Lawrence, M. S., Ho, D. Y. & Sapolsky, R. M. Limitations in the neuroprotective potential of gene therapy with Bcl-2. Brain Res. 859, 202–206 (2000).
Dumas, T. C., McLaughlin, J. R., Ho, D. Y., Lawrence, M. S. & Sapolsky, R. M. Gene therapies that enhance hippocampal neuron survival after an excitotoxic insult are not equivalent in their ability to maintain synaptic transmission. Exp. Neurol. 166, 180–189 (2000).
Myers, K. M. et al. Bcl-2 protects neural cells from cyanide/aglycemia-induced lipid oxidation, mitochondrial injury, and loss of viability. J. Neurochem. 65, 2432–2440 (1995).
Cao, Y. J., Shibata, T. & Rainov, N. G. Hypoxia-inducible transgene expression in differentiated human NT2N neurons — a cell culture model for gene therapy of postischemic neuronal loss. Gene Ther. 8, 1357–1362 (2001).
Linnik, M. D., Zahos, P., Geschwind, M. D. & Federoff, H. J. Expression of bcl-2 from a defective herpes simplex virus-1 vector limits neuronal death in focal cerebral ischemia. Stroke 26, 1670–1675 (1995). Another of the earliest demonstrations of overexpression of a protective gene against a necrotic neurological insult. | Article
Antonawich, F. J., Federoff, H. J. & Davis, J. N. Bcl-2 transduction, using a herpes simplex virus amplicon, protects hippocampal neurons from transient global ischemia. Exp. Neurol. 156, 130–137 (1999).
Shimazaki, K., Urabe, M., Monahan, J., Ozawa, K. & Kawai, N. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 7, 1244–1249 (2000).
Lou, J., Lenke, L., Xu, F. & O'Brien, M. In vivo Bcl-2 oncogene neuronal expression in the rat spinal cord. Spine 23, 517–523 (1998).
Yamada, M. et al. Herpes simplex virus vector-mediated expression of bcl-2 protects spinal motor neurons from degeneration following root avulsion. Exp. Neurol. 168, 225–230 (2001).
Bonfanti L. et al. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J. Neurosci. 16, 4186–4194 (1996).
Matsuoka, N. et al. Adenovirus-mediated gene transfer of Bcl-xL prevents cell death in primary neuronal culture of the rat. Neurosci. Lett. 270, 177–180 (1999).
Blomer, U., Kafri, T., Randolph-Moore, L., Verma, I. M. & Gage, F. H. Bcl-xL protects adult septal cholinergic neurons from axotomized cell death. Proc. Natl Acad. Sci. USA 95, 2603–2608 (1998).
Kugler, S. et al. Neuron-specific expression of therapeutic proteins: evaluation of different cellular promoters in recombinant adenoviral vectors. Mol. Cell. Neurosci. 17, 78–96 (2001).
Simons, M. et al. Adenovirus-mediated gene transfer of inhibitors of apoptosis delays apoptosis of cerebellar granule neurons. J. Neurochem. 72, 292–301 (1999).
Von Coelln, R. et al. Rescue from death but not from functional impairment: caspase inhibition protects dopaminergic cells against 6-OHDA-induced apoptosis but not against the loss of their terminals. J. Neurochem. 77, 263–273 (2001)
Xu, D. et al. Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nature Med. 3, 997–1004 (1997). | Article
Matsuzaki, H. et al. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J. Neurochem. 73, 2037–2046 (1999). | Article
Halterman, M. W., Miller, C. C. & Federoff, H. J. Hypoxia-inducible factor-1α mediates hypoxia-induced delayed neuronal death that involves p53. J. Neurosci. 19, 6818–6824 (1999). | Article
Mochizuki, H. et al. An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc. Natl Acad. Sci. USA 98, 10918–10923 (2001).
McFadden, G. Even viruses can learn to cope with stress. Science 279, 40–41 (1998).
Roy, M., Hom, J. & Sapolsky, R. M. Neuroprotection with herpes simplex viral vectors expressing virally derived anti-apoptotic agents. Brain Res. 901, 12–22 (2001).
Roy, M., Hom, J. J. & Sapolsky, R. M. HSV-mediated delivery of virally derived anti-apoptotic genes protects the rat hippocampus from damage following excitotoxicity but not metabolic disruption. Gene Ther. 9, 214–219 (2002).
Betz, A. L., Yang, G. Y. & Davidson, B. L. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J. Cereb. Blood Flow Metab. 15, 547–551 (1995).
Hagan, P. et al. Adenovirus-mediated over-expression of IL-1 receptor antagonist reduces susceptibility to excitotoxic brain injury in perinatal rats. Neuroscience 75, 1033–1045 (1996).
Yang, G. Y., Zhao, Y. J., Davidson, B. L. & Betz, A. L. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 751, 181–188 (1997).
Pechan, P., Yoshida, T., Panahian, N., Moskowitz, M. A. & Breakefield, X. O. Genetically modified fibroblasts producing NGF protect hippocampal neurons after ischemia in the rat. Neuroreport 6, 669–672 (1995).
Tuszynski, M. H. et al. Grafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelination. Cell Transplant. 7, 187–196 (1998).
Hermann, D. M., Kilic, E., Kugler, S., Isenmann, S. & Bahr M. Adenovirus-mediated glial cell line-derived neurotrophic factor expression protects against subsequent cortical cold injury in rats. Neurobiol. Dis. 8, 964–973 (2001).
Hayashi, K. et al. Gene therapy for preventing neuronal death using hepatocyte growth factor: in vivo gene transfer of HGF to subarachnoid space prevents delayed neuronal death in gerbil hippocampal CA1 neurons. Gene Ther. 8, 1167–1173 (2001).
Gupta, A. et al. Neuroprotective effects of an adenoviral vector expressing the glucose transporter: a detailed description of the mediating cellular events. Brain Res. 908, 49–57 (2001).
Beaucamp, N., Harding, T. C., Geddes, B. J., Williams, J. & Uney, J. B. Overexpression of hsp70i facilitates reactivation of intracellular proteins in neurons and protects them from denaturing stress. FEBS Lett. 441, 215–219 (1998).
Mosser, D. D., Caron, A. W., Bourget, L., Denis-Larose, C. & Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell Biol. 17, 5317–5327 (1997). | Article
Bruey, J. M. et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nature Cell Biol. 2, 645–652 (2000).
Beere, H. & Green, D. Stress management — heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 11, 6–10 (2001).
Kelly, S. et al. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of bcl-2. Ann. Neurol. 52, 160–167 (2002).
Yu, S. et al. Mediation of Poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297, 259–263 (2002).
Kane, D. J., Ord, T., Anton, R. & Bredesen, D. E. Expression of bcl-2 inhibits necrotic neural cell death. J. Neurosci. Res. 40, 269–275 (1995).
Dumas, T. & Sapolsky, R. Gene therapy against neurological insults: sparing neurons versus sparing function. Trends Neurosci. 24, 695–700 (2001).
Chattopadhyay, M. et al. In vivo gene therapy for pyridoxine-induced neuropathy by HSV-mediated gene transfer of neurotrophin-3. Ann. Neurol. 51, 19–27 (2002).
Spencer, B. et al. HSV-1 vector-delivered FGF2 to the retina is neuroprotective but does not preserve functional responses. Mol. Ther. 3, 746–756 (2001).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996). The first report regarding the use of lentiviruses for gene therapy in the nervous system.
Chard, P. S. et al. Regulation of excitatory transmission at hippocampal synapses by calbindin D28K. Proc. Natl Acad. Sci. USA 92, 5144–5148 (1995). A key cautionary paper showing that a gene therapy strategy, while potentially protective against a necrotic insult, can significantly impair normal neuronal function. | Article
Sapolsky, R., Ho, D., McLaughlin, J. The ins and outs of an on and off. Nature biotechnol. 16, 516 (1998).
Lee, H. C., Kim, S. J., Kim, K. S., Shin, H. C. & Yoon, J. W. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature 408, 483–486 (2000).
Ozawa, C. R., Ho, J. J., Tsai, D. J., Ho, D. Y. & Sapolsky, R. M. Neuroprotective potential of a stress-induced viral vector system. Proc. Natl Acad. Sci. USA 97, 9270–9272 (2000). A first demonstration that endocrine stress signals triggered by a necrotic neurological insult can be harnessed to induce expression of protective vectors.
Kassell, N. et al. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J. Neurosurg. 73, 18–36 (1990).
Wolman, R. L. et al. Cerebral injury after cardiac surgery: indication of a group at extraordinary risk. Stroke 30, 514–522 (1999). | Article
Andsberg, G. et al. Neuropathological and behavioral consequences of adeno–associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiol. Dis. 9, 187–204 (2002).
Yagi, T. et al. Rescue of ischemic brain injury by adenoviral gene transfer of glial cell line-derived neurotrophic factor after transient global ischemia in gerbils. Brain Res. 885, 273–282 (2000).
Tsai, T. H. et al. Recombinant adeno-associated virus vector expressing glial cell line-derived neurotrophic factor reduces ischemia-induced damage. Exp. Neurol. 166, 266–275 (2000).
Bemelmans, A. et al. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington's disease, as demonstrated by adenoviral gene transfer. Human Gene Ther. 10, 2987–2997 (1999).
Gravel, C., Gotz, R., Lorrain, A. & Sendtner, M. Adenoviral gene transfer of ciliary neurotrophic factor and brain-derived neurotrophic factor leads to long-term survival of axotomized motor neurons . Nature Med. 3, 765–770 (1997).
Mandel, R. et al. Nerve growth factor expressed in the medial septum following in vivo gene delivery using a recombinant AAV vector protects cholinergic neurons from fimbria-fornix lesion-induced degeneration. Exp. Neurol. 155, 59–64 (1999).
Chen, X., Frisina, R. D., Bowers, W. J., Frisina, D. R. & Federoff, H. J. HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage. Mol. Ther. 3, 958–963 (2001).
Grill, R., Murai, K., Blesch, A., Gage, F. H. & Tuszynski, M. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 17, 5560–5572 (1997). | Article
Acknowledgements
Manuscript assistance was provided by E. Cheng, T. Dumas, D. Kaufer, A. Lee and H. Zhao.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
OMIM
Swiss-Prot
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- AXOTOMY
-
Cutting of the axon.
- APOPTOSIS
-
A common form of cell death, also known as 'intrinsic' or 'programmed' cell death. Many physiological and developmental stimuli cause apoptosis, and the mechanism is used frequently to delete unwanted, superfluous or potentially harmful cells, such as those undergoing transformation. Apoptosis involves cell shrinkage, condensation of chromatin in the periphery of the nucleus, cell-membrane blebbing and DNA fragmentation into multiples of about 180 base pairs. Eventually, the cell breaks up into many membrane-bound 'apoptotic bodies', which are phagocytosed by a neighbouring cell.
- MACROPHAGE
-
Any cell of the mononuclear phagocyte system that is characterized by its ability to phagocytose foreign particulate and colloidal material.
- PARAPTOSIS
-
A form of programmed cell death that does not require the activation of caspases, and produces different changes in cell morphology from those that are usually associated with apoptosis.
- OUTWARDLY-RECTIFYING K+ CHANNELS
-
Potassium channels through which ions flow more easily out of than into the cell.
- AFTERHYPERPOLARIZATION
-
The membrane hyperpolarization that follows the occurrence of an action potential.
- HEAT-SHOCK
-
A cellular response that is caused by exposure to a temperature that is higher than normal. The heat-shock proteins, many of which function as molecular chaperones, are synthesized in larger amounts under these conditions, and many of them are also crucial for cellular functions under non-stress conditions.
- DOMINANT-NEGATIVE MUTANT
-
A mutant molecule that can form a heteromeric complex with the normal molecule, knocking out the activity of the entire complex.
- COMPLEMENT
-
The complement system is a set of plasma proteins that attack extracellular pathogens. The pathogen becomes coated with complement proteins that facilitate pathogen removal by phagocytes. Complement components are also involved in inflammation and tissue destruction.
- NEUTROPHIL
-
A phagocytic cell of the myeloid lineage that has an important role in the inflammatory response, undergoing chemotaxis towards sites of infection or wounding.
- MOLECULAR CHAPERONE
-
A protein that assists in the non-covalent assembly of a protein complex but does not participate in its function.
- ELECTRORETINOGRAMS
-
Measurements of the retinal response to light, typically using an electrode attached to the cornea.
Rights and permissions
About this article
Cite this article
Sapolsky, R. Neuroprotective gene therapy against acute neurological insults. Nat Rev Neurosci 4, 61–69 (2003). https://doi.org/10.1038/nrn1006
Issue Date:
DOI: https://doi.org/10.1038/nrn1006
This article is cited by
-
Characterization of temporal expressions of FOXO and pFOXO proteins in the hippocampus by kainic acid in mice: involvement of NMDA and non-NMDA receptors
Archives of Pharmacal Research (2016)
-
An Insult-Inducible Vector System Activated by Hypoxia and Oxidative Stress for Neuronal Gene Therapy
Translational Stroke Research (2011)
-
Comparison of GAD65 and 67 Immunoreactivity in the Lumbar Spinal Cord Between Young Adult and Aged Dogs
Neurochemical Research (2011)
-
Blocking Glucocorticoid and Enhancing Estrogenic Genomic Signaling Protects against Cerebral Ischemia
Journal of Cerebral Blood Flow & Metabolism (2009)
-
Restructuring the neuronal stress response with anti-glucocorticoid gene delivery
Nature Neuroscience (2004)