Key Points
-
Myxobacteria are Gram-negatives commonly found in the top soil that exhibit social, multicellular behaviour.
-
In the presence of nutrients, myxobacteria feed by forming cooperative swarms of cells, and can prey on other bacteria. In the absence of nutrients, at high cell density on a solid surface they undergo a complex developmental programme, which culminates in the formation of a multicellular fruiting body.
-
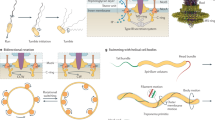
Myxobacteria move by gliding motility, which is controlled by two different gliding engines: the S-engine and the A-engine. The S-engine acts as the 'puller' and comprises pili that pull the cells forward by retraction. The A-engine acts as the 'pusher' and pushes cells forward by secreting ribbons of polysaccharide-rich slime.
-
The multicellular development programme of myxobacteria is controlled by a cell-contact-dependent signal, the C-signal.
-
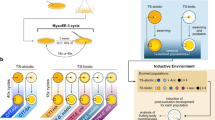
The following development scheme for fruiting-body formation is proposed. The first form of organized movement in a myxobacterial culture is the formation of a wave pattern. The collision of travelling waves of cells in an area of high-cell density creates stationary aggregates of cells, which can become motile and fuse with adjacent aggregates by an as-yet-unknown mechanism. Within the motile aggregates, the myxobacterial cells are streaming in cycles. Travelling waves of cells continue to wash over the aggregates, which accumulate in size to form fruiting bodies comprising up to 105 individual cells. Cell-to-cell contact by motile cells within the aggregates transmits the C-signal between cells, and through a positive-feedback mechanism, the level of C-signal reaches the threshold required for sporulation.
-
The 40 different species of myxobacteria can form fruiting bodies in a variety of shapes and sizes, with both depending on multicellular communication through a cell-contact-dependent system. Understanding this fascinating process could have implications for eukaryotic developmental biology.
Abstract
The myxobacteria are Gram-negative organisms that are capable of multicellular, social behaviour. In the presence of nutrients, swarms of myxobacteria feed cooperatively by sharing extracellular digestive enzymes, and can prey on other bacteria. When the food supply runs low, they initiate a complex developmental programme that culminates in the production of a fruiting body. Myxobacteria move by gliding and have two, polarly positioned engines to control their motility. The two engines undergo coordinated reversals, and changes in the reversal frequency and speed are responsible for the different patterns of movement that are seen during development. The myxobacteria communicate with each other and coordinate their movements through a cell-contact-dependent signal. Here, the cell movements that culminate in the development of the multicellular fruiting body are reviewed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Reichenbach, H. in The Myxobacteria (ed. Rosenberg, E.) 1–50 (Springer–Verlag, New York, 1984).
Rosenberg, E., Keller, K. H. & Dworkin, M. Cell density-dependent growth of Myxococcus xanthus on casein. J. Bacteriol. 129, 770–777 (1977).
Reichenbach, H. in Myxobacteria II (eds Dworkin, M. & Kaiser, D.) 13–62 (ASM Press, Washington DC, 1993).
Burchard, R. P. Gliding motility mutants of Myxococcus xanthus. J. Bacteriol. 104, 940–947 (1970).
Kaiser, D. in The Myxobacteria (ed. Rosenberg, E.) 163–184 (Springer–Verlag, New York, 1984).
Hagen, D. C., Bretscher, A. P. & Kaiser, D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64, 284–296 (1978).
Kim, S. K. & Kaiser, D. C-factor: a cell-cell signalling protein required for fruiting-body morphogenesis of M. xanthus. Cell 61, 19–26 (1990).
Kuspa, A., Plamann, L. & Kaiser, D. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 174, 3319–3326 (1992).
Plamann, L., Kuspa, A. & Kaiser, D. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J. Bacteriol. 174, 3311–3318 (1992).
Kim, S. K. & Kaiser, D. Purification and properties of Myxococcus xanthus C-factor, an intercellular signaling protein. Proc. Natl Acad. Sci. USA 87, 3635–3639 (1990).
Søgaard-Andersen, L. & Kaiser, D. C-factor, a cell-surface-associated intercellular signaling protein, stimulates the cytoplasmic Frz signal transduction system in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 93, 2675–2679 (1996).
Søgaard-Andersen, L. Coupling gene expression and multicellular morphogenesis during fruiting body formation in Myxococcus xanthus. Mol. Microbiol. 48, 1–8 (2003).
Kim, S. K. & Kaiser, D. Cell alignment required in differentiation of Myxococcus xanthus. Science 249, 926–928 (1990).
McBride, M. J., Hartzell, P. & Zusman, D. R. in Myxobacteria II (eds Dworkin, M. & Kaiser, D.) 285–305 (ASM Press, Washington DC, 1993).
Hodgkin, J. & Kaiser, D. Genetics of gliding motility in M. xanthus (Myxobacterales): genes controlling movement of single cells. Mol. Gen. Genet. 171, 167–176 (1979).
Hodgkin, J. & Kaiser, D. Genetics of gliding motility in M. xanthus (Myxobacterales): two gene systems control movement. Mol. Gen. Genet. 171, 177–191 (1979).
Maier, B., Potter, L., So, M., Seifert, H. S. & Sheetz, M. P. Single pilus motor forces exceed 100 pN. Proc. Natl Acad. Sci. USA 99, 16012–16017 (2002).
Kaiser, D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 76, 5952–5956 (1979).
Wu, S. S. & Kaiser, D. Regulation of expression of the pilA gene in Myxococcus xanthus. J. Bacteriol. 179, 7748–7758 (1997).
Wall, D., Kolenbrander, P. E. & Kaiser, D. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pili biogenesis, S motility and development. J. Bacteriol. 181, 24–33 (1999).
Wolfgang, M. et al. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29, 321–330 (1998).
Vale, R. D. AAA proteins: lords of the ring. J. Cell Biol. 150, F13–F19 (2000).
Merz, A. J., So, M. & Sheetz, M. P. Pilus retraction powers bacterial twitching motility. Nature 407, 98–102 (2000). Describes the use of laser tweezers to directly measure the force of the type IV pilus retraction.
Merz, A. J. & Forest, K. T. Bacterial surface motility: slime trails, grappling hooks and nozzles. Curr. Biol. 12, R297–R303 (2002).
Sun, H., Zusman, D. R. & Shi, W. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 10, 1143–1146 (2000).
Kaiser, D. How do pili pull? Curr. Biol. 10, R777–R780 (2000).
Skerker, J. & Berg, H. Direct observation of extension and retraction of type IV pili. Proc. Natl Acad. Sci. USA 98, 6901–6904 (2001).
Wall, D. & Kaiser, D. Type IV pili and cell motility. Mol. Microbiol. 32, 1–10 (1999).
Arnold, J. W. & Shimkets, L. J. Cell surface properties correlated with cohesion in Myxococcus xanthus. J. Bacteriol. 170, 5771–5777 (1988).
Arnold, J. W. & Shimkets, L. Inhibition of cell–cell interactions in Myxococcus xanthus by congo red. J. Bacteriol. 170, 5765–5770 (1988).
Behmlander, R. M. & Dworkin, M. Biochemical and structural analyses of the extracellular matrix fibrils of Myxococcus xanthus. J. Bacteriol. 176, 6295–6303 (1994).
Dworkin, M. Fibrils as extracellular appendages of bacteria: their role in contact-mediated cell–cell interactions in Myxococcus xanthus. BioEssays 21, 590–595 (1999).
Yang, Z. et al. The Myxococcus xanthus dif genes are required for the biogenesis of cell surface fibrils essential for social gliding motility. J. Bacteriol. 182, 5793–5798 (2000).
Kaiser, D. & Crosby, C. Cell movement and its coordination in swarms of Myxococcus xanthus. Cell Motil. 3, 227–245 (1983).
Bowden, M. G. & Kaplan, H. B. The Myxococcus xanthus lipopolysaccharide O-antigen is required for social motility and multicellular development. Mol. Microbiol. 30, 275–284 (1998).
Li, Y. et al. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc. Natl Acad. Sci. USA 100, 5443–5448 (2003).
Kuhlwein, H. & Reichenbach, H. Schwarmentwicklung und Morphogenese bei Myxobacterien–Archangium, Myxococcus, Chondrococcus, Chondromyces. (eds Heunert, H. H. & Kuczka, H.) Film C893 (Inst. Wissensch. Film, Gottingen, Germany, 1965).
Burchard, R. P. Trail following by gliding bacteria. J. Bacteriol. 152, 495–501 (1982).
Shi, W. & Zusman, D. R. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc. Natl Acad. Sci. USA 90, 3378–3382 (1993).
Wolgemuth, C., Hoiczyk, E., Kaiser, D. & Oster, G. How myxobacteria glide. Curr. Biol. 12, 1–20 (2002). Provides experimental evidence for the secretion of slime from jets at the rear of M. xanthus cells that propels them for A-motility.
Hoiczyk, E. & Baumeister, W. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr. Biol. 8, 1161–1168 (1998).
Stanier, R. Y. Elasticotaxis in myxobacteria. J. Bacteriol. 44, 405–412 (1942).
Fontes, M. & Kaiser, D. Myxococcus cells respond to elastic forces in their substrate. Proc. Natl Acad. Sci. USA 96, 8052–8057 (1999).
Dworkin, M. Tactic behavior of Myxococcus xanthus. J. Bacteriol. 154, 452–459 (1983).
Burchard, R. P. Growth of surface colonies of the gliding bacterium Myxococcus xanthus. Arch. Microbiol. 96, 247–254 (1974).
Sager, B. & Kaiser, D. Intercellular C-signaling and the traveling waves of Myxococcus. Genes Dev. 8, 2793–2804 (1994).
Kim, S. K. & Kaiser, D. Cell motility is required for the transmission of C-factor, an intercellular signal that coordinates fruiting body morphogenesis of Myxococcus xanthus. Genes Dev. 4, 896–905 (1990).
Gronewold, T. M. A. & Kaiser, D. The act operon controls the level and time of C-signal production for M. xanthus development. Mol. Microbiol. 40, 744–756 (2001). Provides evidence for a positive feedback circuit that increases the number of C-signal molecules that are displayed on the cell surface.
Gronewold, T. M. A. & Kaiser, D. act operon control of developmental gene expression in Myxococcus xanthus. J. Bacteriol. 184, 1172–1179 (2002).
Ogawa, M., Fujitani, S., Mao, X., Inouye, S. & Komano, T. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22, 757–767 (1996).
Ellehauge, E., Norregaard-Madsen, M. & Søgaard-Andersen, L. The FruA signal transduction protein provides a checkpoint for the temporal coordination of intercellular signals in M. xanthus development. Mol. Microbiol. 30, 807–813 (1998). References 50 and 51 show that the fruA response-regulator links reception of the C-signal to the frz chemosensory pathway.
Søgaard-Andersen, L., Slack, F., Kimsey, H. & Kaiser, D. Intercellular C-signaling in Myxococcus xanthus involves a branched signal transduction pathway. Genes Dev. 10, 740–754 (1996).
McBride, M. J., Weinberg, R. A. & Zusman, D. R. Frizzy aggregation genes of the gliding bacterium Myxococcus xanthus show sequence similarities to the chemotaxis genes of enteric bacteria. Proc. Natl Acad. Sci. USA 86, 424–428 (1989).
McCleary, W. R., McBride, M. J. & Zusman, D. R. Developmental sensory transduction in Myxococcus xanthus involves methylation and demethylation of frzCD. J. Bacteriol. 172, 4877–4887 (1990).
Welch, R. & Kaiser, D. Cell behavior in traveling wave patterns of myxobacteria. Proc. Natl Acad. Sci. USA 98, 14907–14912 (2001).
Reichenbach, H. Rhythmic motion in swarms of myxobacteria. Ber. Deutsch. Bot. Ges. 78, 102–105 (1965).
Shimkets, L. & Kaiser, D. Induction of coordinated movement of Myxococcus xanthus cells. J. Bacteriol. 152, 451–461 (1982).
Igoshin, O., Mogilner, A., Welch, R., Kaiser, D. & Oster, G. Pattern formation and traveling waves in myxobacteria: theory and modeling. Proc. Natl Acad. Sci. USA 98, 14913–14918 (2001).
Kuner, J. & Kaiser, D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J. Bacteriol. 151, 458–461 (1982).
Jelsbak, L. & Søgaard-Andersen, L. The cell-surface associated C-signal induces behavioral changes in individual M. xanthus cells during fruiting body morphogenesis. Proc. Natl Acad. Sci. USA 96, 5031–5036 (1999).
Jelsbak, L. & Søgaard-Andersen, L. Pattern formation by a cell-surface associated morphogen in M. xanthus. Proc. Natl Acad. Sci. USA 99, 2032–2037 (2002). The first cell-tracking experiments of cells that are induced to stream by the C-signal.
Sager, B. & Kaiser, D. Two cell-density domains within the Myxococcus xanthus fruiting body. Proc. Natl Acad. Sci. USA 90, 3690–3694 (1993).
O'Connor, K. A. & Zusman, D. R. Patterns of cellular interactions during fruiting-body formation in Myxococcus xanthus. J. Bacteriol. 171, 6013–6024 (1989)
Julien, B., Kaiser, D. & Garza, A. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 97, 9098–9103 (2000). The first paper to show that the C-signal induces sporulation only inside of a fruiting body.
O'Connor, K. A. & Zusman, D. R. Behavior of peripheral rods and their role in the life cycle of Myxococcus xanthus. J. Bacteriol. 173, 3342–3355 (1991).
Kaiser, D. Cell fate and organogenesis in bacteria. Trends Genet. 15, 273–277 (1999).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Henrichsen, J. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36, 478–503 (1972).
Henrichsen, J. Twitching motility and its mechanism. Acta Path. Microbiol. Scand. 83, B187–B190 (1975).
Mattick, J. S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56, 289–314 (2002).
Bieber, D. et al. Type IV pili, transient bacterial aggregates and virulence in enteropathogenic Escherichia coli. Science 280, 2114–2118 (1998).
O'Toole, G. A. & Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28, 449–461 (1998).
Klausen, M. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48, 1511–1524 (2003).
Börner, U., Deutsch, A., Reichenbach, H. & Bär, M. Rippling patterns in aggregates of myxobacteria arise from cell–cell collisions. Phys. Rev. Lett. 89, 078101 (2002).
Alber, M. S., Jiang, Y. & Kiskowski, M. Lattice gas cellular automata model for rippling and aggregation in myxobacteria. SIAM J. Appl. Math. (in the press).
Acknowledgements
I thank L. Jelsbak, O. Igoshin, G. Oster and M. Alber for their suggestions. D. K. is supported by a grant from the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Supplementary information
Online Movie
To view this movie you need Quicktime. To download this player for free click here: Download Quicktime player . (MOV 631 kb)
Related links
Glossary
- SPORANGIUM
-
A specialized structure that contains myxobacterial spores.
- TRANSDUCTION
-
The virus-mediated transfer of host DNA (plasmid or chromosomal) from a donor cell to a recipient cell.
- TRANSFECTION
-
The transformation of prokaryotic cells with viral DNA or RNA.
- TYPE IV PILI
-
Elongated hair-like structures extending from the surface of Gram-negative cells that are independent of flagella, and which can retract and pull the cell forward.
- AAA MOTOR PROTEIN
-
An ATPase that is associated with various cellular activities. AAA proteins are essential in all organisms.
- FIBRILS
-
Filamentous extracellular matrix material comprising polysaccharides and protein.
- O-ANTIGEN
-
A heat-stable antigen that is associated with Gram-negative bacteria and which comprises chains of identical oligosaccharide units that can vary in length.
- QUORUM SENSOR
-
An extracellular signal molecule, the concentration of which is proportional to the cell concentration, and which is used by many bacteria to detect cell density
- FOURIER ANALYSIS
-
Mathematical decomposition of a complex periodic function into a sum of simple sine waves.
Rights and permissions
About this article
Cite this article
Kaiser, D. Coupling cell movement to multicellular development in myxobacteria. Nat Rev Microbiol 1, 45–54 (2003). https://doi.org/10.1038/nrmicro733
Issue Date:
DOI: https://doi.org/10.1038/nrmicro733
This article is cited by
-
Multi-scale dynamic imaging reveals that cooperative motility behaviors promote efficient predation in bacteria
Nature Communications (2023)
-
Spatiotemporal distribution of chemical signatures exhibited by Myxococcus xanthus in response to metabolic conditions
Analytical and Bioanalytical Chemistry (2022)
-
Topological defects promote layer formation in Myxococcus xanthus colonies
Nature Physics (2021)
-
The evolution of convex trade-offs enables the transition towards multicellularity
Nature Communications (2021)
-
A statistical physics view of swarming bacteria
Movement Ecology (2019)