Key Points
-
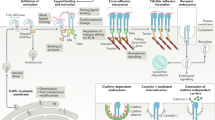
The aim of this Review is to discuss how molecular research into the complex interplay between cell adhesion and the cytoskeleton, combined with advanced surface nanoengineering technologies, can shed light on the mechanisms by which cells sense the neighbouring microenvironment and nanoenvironment.
-
Cells demonstrate an extraordinary capacity to respond to a wide range of features of the surrounding matrix, including its chemical nature and physical properties.
-
Contemporary methods of microfabrication and nanofabrication enable the production of substrates with well-defined topography, rigidity, ligand spacing and anisotropy. Plating cells on surfaces with diverse physical properties has revealed the exquisite capacity of cells to sense, and differentially respond, to such adhesive matrices.
-
Mechanical modulation of the various adhesion complexes leads to the generation of integrin-mediated signals that affect multiple features of cell shape, activity and fate.
-
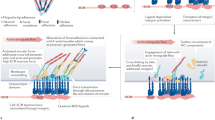
The mechanosensitivity of integrin-based adhesions (focal adhesions) is attributable to the complexity and modularity of the adhesion complexes, and their different functional modules. Specifically, mechanosensitive elements can be found in essentially every structural–functional module of the adhesion sites, including the extracellular matrix itself, the integrin receptors, the actin-linking and actin polymerization machinery, and the signal-generating and transducing modules.
-
Owing to the overall, combined mechanosensitivity of focal adhesions, cytoskeleton-generated forces affect the initiation, maturation and further growth of these structures.
-
In turn, the pre-existing integrin adhesions determine the organization of the actin cytoskeleton by creating boundary conditions that determine the spatial organization of the cytoskeleton; by inducing actin polymerization at the local level; and by global signalling, mainly through the Rho pathways, thereby regulating the overall assembly of the actin-containing structures.
-
Focal adhesions seem to be responsible for the spatio-temporal coordination of the multiple signalling events that are triggered by cell–extracellular matrix interactions.
Abstract
Recent progress in the design and application of artificial cellular microenvironments and nanoenvironments has revealed the extraordinary ability of cells to adjust their cytoskeletal organization, and hence their shape and motility, to minute changes in their immediate surroundings. Integrin-based adhesion complexes, which are tightly associated with the actin cytoskeleton, comprise the cellular machinery that recognizes not only the biochemical diversity of the extracellular neighbourhood, but also its physical and topographical characteristics, such as pliability, dimensionality and ligand spacing. Here, we discuss the mechanisms of such environmental sensing, based on the finely tuned crosstalk between the assembly of one type of integrin-based adhesion complex, namely focal adhesions, and the forces that are at work in the associated cytoskeletal network owing to actin polymerization and actomyosin contraction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Geiger, B. & Bershadsky, A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell 110, 139–142 (2002).
Bershadsky, A. D., Balaban, N. Q. & Geiger, B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19, 677–695 (2003).
Chen, C. S. Mechanotransduction — a field pulling together? J. Cell Sci. 121, 3285–3292 (2008).
Curtis, A. & Riehle, M. Tissue engineering: the biophysical background. Phys. Med. Biol. 46, R47–R65 (2001).
Spatz, J. P. & Geiger, B. Molecular engineering of cellular environments: cell adhesion to nano-digital surfaces. Methods Cell Biol. 83, 89–111 (2007).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nature Rev. Mol. Cell Biol. 7, 265–275 (2006).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Thery, M. et al. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl Acad. Sci. USA 103, 19771–19776 (2006). Shapes of adhesive islands determine the localization of lamellipodial extensions and the organization of the microtubule system in the attached cells.
Xia, N. et al. Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation. FASEB J. 22, 1649–1659 (2008).
Lock, J. G., Wehrle-Haller, B. & Stromblad, S. Cell–matrix adhesion complexes: master control machinery of cell migration. Semin. Cancer Biol. 18, 65–76 (2008).
Delon, I. & Brown, N. H. Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 19, 43–50 (2007).
Tilghman, R. W. & Parsons, J. T. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin. Cancer Biol. 18, 45–52 (2008).
Berrier, A. L. & Yamada, K. M. Cell–matrix adhesion. J. Cell. Physiol. 213, 565–573 (2007).
Bershadsky, A., Kozlov, M. & Geiger, B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr. Opin. Cell Biol. 18, 472–481 (2006).
Zaidel-Bar, R., Itzkovitz, S., Ma'ayan, A., Iyengar, R. & Geiger, B. Functional atlas of the integrin adhesome. Nature Cell Biol. 9, 858–867 (2007). Bioinformatics analysis of all available experimental data revealed functional interactions between proteins that form integrin-mediated adhesion complexes and unravelled the prevalent network motifs in the protein interaction map.
Giannone, G. & Sheetz, M. P. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 16, 213–223 (2006).
Nayal, A., Webb, D. J. & Horwitz, A. F. Talin: an emerging focal point of adhesion dynamics. Curr. Opin. Cell Biol. 16, 94–98 (2004).
Hersel, U., Dahmen, C. & Kessler, H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415 (2003).
Arnold, M. et al. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 5, 383–388 (2004).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Dalby, M. J., Riehle, M. O., Johnstone, H., Affrossman, S. & Curtis, A. S. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials 23, 2945–2954 (2002).
Chen, C. S., Tan, J. & Tien, J. Mechanotransduction at cell–matrix and cell–cell contacts. Annu. Rev. Biomed. Eng. 6, 275–302 (2004).
Rumpler, M., Woesz, A., Dunlop, J. W., van Dongen, J. T. & Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface (2008).
Cukierman, E., Pankov, R., Stevens, D. R. & Yamada, K. M. Taking cell–matrix adhesions to the third dimension. Science 294, 1708–1712 (2001).
Blummel, J. et al. Protein repellent properties of covalently attached PEG coatings on nanostructured SiO2-based interfaces. Biomaterials 28, 4739–4747 (2007).
Elbert, D. L. & Hubbell, J. A. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules 2, 430–441 (2001).
Maheshwari, G., Brown, G., Lauffenburger, D. A., Wells, A. & Griffith, L. G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113, 1677–1686 (2000).
Mrksich, M., Dike, L. E., Tien, J., Ingber, D. E. & Whitesides, G. M. Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Exp. Cell Res. 235, 305–313 (1997).
Roberts, C. et al. Using mixed self-assembled monolayers presenting RGD and (EG)3OH groups to characterize long-term attachment of bovine capillary endothelial cells to surfaces J. Am. Chem. Soc. 120, 6548–6555 (1998).
Massia, S. P. & Hubbell, J. A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 114, 1089–1100 (1991).
Fratzl, P. et al. Fibrillar structure and mechanical properties of collagen. J. Struct. Biol. 122, 119–122 (1998).
Jiang, F., Horber, H., Howard, J. & Muller, D. J. Assembly of collagen into microribbons: effects of pH and electrolytes. J. Struct. Biol. 148, 268–278 (2004).
Meller, D., Peters, K. & Meller, K. Human cornea and sclera studied by atomic force microscopy. Cell Tissue Res. 288, 111–118 (1997).
Glass, R. et al. Micro-nanostructured interfaces by inorganic block copolymer micellar monolayers as negative resist for electron-beam lithography. Adv. Funct. Mater. 13, 569–575 (2003).
Glass, R. et al. Block copolymer micelle nanolithography on non-conductive substrates. New J. Phys. 6, 101 (2004).
Glass, R., Möller, M. & Spatz, J. P. Micellar nanolithography. Nanotechnology 14, 1153–1160 (2003).
Spatz, J. P. et al. Metal and metaloxide nanodot pattern by means of a diblock copolymer template. Langmuir 16, 407–415 (2000).
Cavalcanti-Adam, E. A. et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 92, 2964–2974 (2007).
Arnold, M. et al. Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing. Nano Lett. 8, 2063–2069 (2008). References 39 and 40 were the first rigorous analyses of the differential cellular response to the spacing of integrin ligands on a substrate.
Smith, M. L. et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 5, e268 (2007). Fluorescence resonance energy transfer analysis revealed that cell-generated mechanical forces induce unfolding of type III modules in the mechanosensory matrix protein fibronectin.
Little, W. C., Smith, M. L., Ebneter, U. & Vogel, V. Assay to mechanically tune and optically probe fibrillar fibronectin conformations from fully relaxed to breakage. Matrix Biol. 27, 451–461 (2008).
Collin, O. et al. Self-organized podosomes are dynamic mechanosensors. Curr. Biol. 18, 1288–1294 (2008).
Alexander, N. R. et al. Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol. 18, 1295–1299 (2008).
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane extracellular matrix–cytoskeleton crosstalk. Nature Rev. Mol. Cell Biol. 2, 793–805 (2001).
Brown, N. H. et al. Talin is essential for integrin function in Drosophila. Dev. Cell 3, 569–579 (2002).
Zervas, C. G., Gregory, S. L. & Brown, N. H. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152, 1007–1018 (2001).
Clark, K. A., McGrail, M. & Beckerle, M. C. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130, 2611–2621 (2003).
Torgler, C. N. et al. Tensin stabilizes integrin adhesive contacts in Drosophila. Dev. Cell 6, 357–369 (2004).
Loer, B. et al. The NHL-domain protein Wech is crucial for the integrin–cytoskeleton link. Nature Cell Biol. 10, 422–428 (2008).
Monkley, S. J., Pritchard, C. A. & Critchley, D. R. Analysis of the mammalian talin2 gene TLN2. Biochem. Biophys. Res. Commun. 286, 880–885 (2001).
Zhang, X. et al. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nature Cell Biol. 10, 1062–1068 (2008). Depletion of both talin 1 and talin 2 prevents the formation of focal adhesions, but permits the formation of unstable lamellipodial extensions in spreading cells.
Montanez, E. et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325–1330 (2008).
Moser, M., Nieswandt, B., Ussar, S., Pozgajova, M. & Fassler, R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nature Med. 14, 325–330 (2008).
Ma, Y. Q., Qin, J., Wu, C. & Plow, E. F. Kindlin-2 (Mig-2): a co-activator of β3 integrins. J. Cell Biol. 181, 439–446 (2008). References 53–55 clearly demonstrate that kindlin 2 or kindlin 3 are required as co-activators of integrin working in concert with talin.
Tanentzapf, G., Martin-Bermudo, M. D., Hicks, M. S. & Brown, N. H. Multiple factors contribute to integrin–talin interactions in vivo. J. Cell Sci. 119, 1632–1644 (2006).
Smith, S. J. & McCann, R. O. A C-terminal dimerization motif is required for focal adhesion targeting of Talin1 and the interaction of the Talin1 I/LWEQ module with F-actin. Biochemistry 46, 10886–10898 (2007).
Gingras, A. R. et al. The structure of the C-terminal actin-binding domain of talin. EMBO J. 27, 458–469 (2008).
Jiang, G., Giannone, G., Critchley, D. R., Fukumoto, E. & Sheetz, M. P. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424, 334–337 (2003).
Humphries, J. D. et al. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 179, 1043–1057 (2007).
Galbraith, C. G., Yamada, K. M. & Sheetz, M. P. The relationship between force and focal complex development. J. Cell Biol. 159, 695–705 (2002).
Alexandrova, A. Y. et al. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 3, e3234 (2008). Focal complexes (nascent adhesions) are formed underneath the lamellipodial extensions and subsequently determine the formation of a boundary between lamellipodia and lamella.
Choi, C. K. et al. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nature Cell Biol. 10, 1039–1050 (2008). Nascent adhesions are formed underneath the lamellipodial extensions, and early stages of their maturation depend on the crosslinking activity of α-actinin and myosin II.
Nobes, C. D. & Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Shroff, H., Galbraith, C. G., Galbraith, J. A. & Betzig, E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nature Methods 5, 417–423 (2008).
Shroff, H. et al. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc. Natl Acad. Sci. USA 104, 20308–20313 (2007).
Giannone, G. et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 128, 561–575 (2007).
Ponti, A., Machacek, M., Gupton, S. L., Waterman-Storer, C. M. & Danuser, G. Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782–1786 (2004).
Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003).
Small, J. V., Stradal, T., Vignal, E. & Rottner, K. The lamellipodium: where motility begins. Trends Cell Biol. 12, 112–120 (2002).
Borisy, G. G. & Svitkina, T. M. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104–112 (2000).
Cramer, L. P. Molecular mechanism of actin-dependent retrograde flow in lamellipodia of motile cells. Front. Biosci. 2, d260–d270 (1997).
Vallotton, P., Danuser, G., Bohnet, S., Meister, J. J. & Verkhovsky, A. B. Tracking retrograde flow in keratocytes: news from the front. Mol. Biol. Cell 16, 1223–1231 (2005).
Vallotton, P., Gupton, S. L., Waterman-Storer, C. M. & Danuser, G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc. Natl Acad. Sci. USA 101, 9660–9665 (2004).
Cai, Y. et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 91, 3907–3920 (2006). Myosin IIA, rather than myosin IIB, is responsible for overall cell contractility and retrograde flow in lamella.
Hu, K., Ji, L., Applegate, K. T., Danuser, G. & Waterman-Storer, C. M. Differential transmission of actin motion within focal adhesions. Science 315, 111–115 (2007). Retrograde actin flow induces the correlated centripetal movement of focal adhesion proteins, thereby revealing a hierarchy in their association with non-mobile integrins.
Guo, W. H. & Wang, Y. L. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol. Biol. Cell 18, 4519–4527 (2007).
Galbraith, C. G., Yamada, K. M. & Galbraith, J. A. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 315, 992–995 (2007). Spatial correlation between integrin activation and actin polymerization in lamellipodia and filopodia is revealed.
Grosheva, I. et al. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp. Eye Res. 82, 945–958 (2006).
Riveline, D. et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186 (2001).
Vicente-Manzanares, M., Zareno, J., Whitmore, L., Choi, C. K. & Horwitz, A. F. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 176, 573–580 (2007). This study, along with references 75, 92, 93 and 95, established that myosin IIA and myosin IIB have different roles in the organization of cell adhesion and motility.
Zaidel-Bar, R., Milo, R., Kam, Z. & Geiger, B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell–matrix adhesions. J. Cell Sci. 120, 137–148 (2007).
Ballestrem, C. et al. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J. Cell Sci. 119, 866–875 (2006).
Zamir, E., Geiger, B. & Kam, Z. Quantitative multicolor compositional imaging resolves molecular domains in cell–matrix adhesions. PLoS ONE 3, e1901 (2008).
Cluzel, C. et al. The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J. Cell Biol. 171, 383–392 (2005).
Ballestrem, C., Hinz, B., Imhof, B. A. & Wehrle-Haller, B. Marching at the front and dragging behind: differential αvβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 155, 1319–1332 (2001).
Pellegrin, S. & Mellor, H. Actin stress fibres. J. Cell Sci. 120, 3491–3499 (2007).
Kumar, S. et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90, 3762–3773 (2006).
Peterson, L. J. et al. Simultaneous stretching and contraction of stress fibers in vivo. Mol. Biol. Cell 15, 3497–3508 (2004).
Katoh, K. et al. Rho-kinase-mediated contraction of isolated stress fibers. J. Cell Biol. 153, 569–584 (2001).
Balaban, N. Q. et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biol. 3, 466–472 (2001).
Even-Ram, S. et al. Myosin IIA regulates cell motility and actomyosin–microtubule crosstalk. Nature Cell Biol. 9, 299–309 (2007). Myosin IIA, rather than myosin IIB, is required for focal adhesion maturation. However, in some situations (microtubule disruption), myosin IIB-dependent focal adhesion maturation can occur.
Vicente-Manzanares, M., Koach, M. A., Whitmore, L., Lamers, M. L. & Horwitz, A. F. Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 183, 543–554 (2008). Myosin IIB is required for the formation of stress fibres and focal adhesions at the cell rear.
Meshel, A. S., Wei, Q., Adelstein, R. S. & Sheetz, M. P. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nature Cell Biol. 7, 157–164 (2005).
Sandquist, J. C. & Means, A. R. The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells. Mol. Biol. Cell 19, 5156–5167 (2008). Differential localization of myosin IIA and IIB depends on a specific sequence at the C terminus.
Gingras, A. R. et al. Structural and dynamic characterization of a vinculin binding site in the talin rod. Biochemistry 45, 1805–1817 (2006).
Papagrigoriou, E. et al. Activation of a vinculin-binding site in the talin rod involves rearrangement of a five-helix bundle. EMBO J. 23, 2942–2951 (2004).
Hytonen, V. P. & Vogel, V. How force might activate talin's vinculin binding sites: SMD reveals a structural mechanism. PLoS Comput. Biol. 4, e24 (2008).
Lee, S. E., Kamm, R. D. & Mofrad, M. R. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. J. Biomech. 40, 2096–2106 (2007).
Johnson, C. P., Tang, H. Y., Carag, C., Speicher, D. W. & Discher, D. E. Forced unfolding of proteins within cells. Science 317, 663–666 (2007).
Vogel, V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 35, 459–488 (2006).
Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006). A novel mechanosensory mechanism that is based on stretch-induced opening of a phosphorylation site in p130CAS is described.
Puklin-Faucher, E., Gao, M., Schulten, K. & Vogel, V. How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J. Cell Biol. 175, 349–60 (2006).
Hayakawa, K., Tatsumi, H. & Sokabe, M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J. Cell Sci. 121, 496–503 (2008). Pulling force applied through stress fibres can locally activate mechanosensitive channels.
Shemesh, T., Geiger, B., Bershadsky, A. D. & Kozlov, M. M. Focal adhesions as mechanosensors: a physical mechanism. Proc. Natl Acad. Sci. USA 102, 12383–12388 (2005).
Ingber, D. E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827 (2006).
Bershadsky, A. D. et al. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 85, 165–173 (2006).
Thery, M., Pepin, A., Dressaire, E., Chen, Y. & Bornens, M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell. Motil. Cytoskeleton 63, 341–355 (2006).
Lehnert, D. et al. Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 117, 41–52 (2004).
Parker, K. K. et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 16, 1195–1204 (2002).
Wang, Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J. Cell Biol. 101, 597–602 (1985).
Endlich, N., Otey, C. A., Kriz, W. & Endlich, K. Movement of stress fibers away from focal adhesions identifies focal adhesions as sites of stress fiber assembly in stationary cells. Cell. Motil. Cytoskeleton 64, 966–976 (2007).
Hotulainen, P. & Lappalainen, P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173, 383–394 (2006). This study, together with reference 112, demonstrates stress fibre growth from a focal adhesion based on local actin polymerization.
Gupton, S. L., Eisenmann, K., Alberts, A. S. & Waterman-Storer, C. M. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J. Cell Sci. 120, 3475–3487 (2007).
Butler, B., Gao, C., Mersich, A. T. & Blystone, S. D. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr. Biol. 16, 242–251 (2006). Formin, rather than Arp2/3, is responsible for the nucleation of actin polymerization by integrin-based adhesion complexes.
Takeya, R., Taniguchi, K., Narumiya, S. & Sumimoto, H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 27, 618–628 (2008).
Kozlov, M. M. & Bershadsky, A. D. Processive capping by formin suggests a force-driven mechanism of actin polymerization. J. Cell Biol. 167, 1011–1017 (2004).
Hirata, H., Tatsumi, H. & Sokabe, M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J. Cell Sci. 121, 2795–2804 (2008). Reveals the involvement of zyxin in the force-dependent actin polymerization from the focal adhesion.
Yoshigi, M., Hoffman, L. M., Jensen, C. C., Yost, H. J. & Beckerle, M. C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 171, 209–215 (2005).
Lele, T. P. et al. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell. Physiol. 207, 187–194 (2006).
Hoffman, L. M. et al. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J. Cell Biol. 172, 771–782 (2006).
Legate, K. R., Montanez, E., Kudlacek, O. & Fassler, R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nature Rev. Mol. Cell Biol. 7, 20–31 (2006).
Moissoglu, K. & Schwartz, M. A. Integrin signalling in directed cell migration. Biol. Cell 98, 547–555 (2006).
Wiesner, S., Legate, K. R. & Fassler, R. Integrin–actin interactions. Cell. Mol. Life Sci. 62, 1081–1099 (2005).
Burridge, K. & Wennerberg, K. Rho and Rac take center stage. Cell 116, 167–179 (2004).
Lu, M. & Ravichandran, K. S. Dock180–ELMO cooperation in Rac activation. Methods Enzymol. 406, 388–402 (2006).
Zaidel-Bar, R., Kam, Z. & Geiger, B. Polarized downregulation of the paxillin–p130CAS–Rac1 pathway induced by shear flow. J. Cell Sci. 118, 3997–4007 (2005).
Dubash, A. D. et al. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J. Cell Sci. 120, 3989–3998 (2007).
Lim, Y. et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 180, 187–203 (2008).
Bass, M. D. et al. p190RhoGAP is the convergence point of adhesion signals from α5β1 integrin and syndecan-4. J. Cell Biol. 181, 1013–1026 (2008).
Arthur, W. T., Petch, L. A. & Burridge, K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719–722 (2000).
Hildebrand, J. D., Taylor, J. M. & Parsons, J. T. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol. Cell. Biol. 16, 3169–3178 (1996).
Schober, M. et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 176, 667–680 (2007).
Danen, E. H., Sonneveld, P., Brakebusch, C., Fassler, R. & Sonnenberg, A. The fibronectin-binding integrins α5β1 and αvβ3 differentially modulate RhoA–GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 159, 1071–1086 (2002).
Danen, E. H. et al. Integrins control motile strategy through a Rho–cofilin pathway. J. Cell Biol. 169, 515–526 (2005).
Katz, B. Z. et al. Physical state of the extracellular matrix regulates the structure and molecular composition of cell–matrix adhesions. Mol. Biol. Cell 11, 1047–1060 (2000).
McCann, R. O. & Craig, S. W. The I/LWEQ module: a conserved sequence that signifies F-actin binding in functionally diverse proteins from yeast to mammals. Proc. Natl Acad. Sci. USA 94, 5679–5684 (1997).
Calderwood, D. A. et al. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl Acad. Sci. USA 100, 2272–2277 (2003).
Garcia-Alvarez, B. et al. Structural determinants of integrin recognition by talin. Mol. Cell 11, 49–58 (2003).
Tadokoro, S. et al. Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 (2003).
Calderwood, D. A. Integrin activation. J. Cell Sci. 117, 657–666 (2004).
Tanentzapf, G. & Brown, N. H. An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nature Cell Biol. 8, 601–606 (2006).
Wegener, K. L. et al. Structural basis of integrin activation by talin. Cell 128, 171–182 (2007).
Banno, A. & Ginsberg, M. H. Integrin activation. Biochem. Soc. Trans. 36, 229–234 (2008).
Ussar, S., Wang, H. V., Linder, S., Fassler, R. & Moser, M. The kindlins: subcellular localization and expression during murine development. Exp. Cell Res. 312, 3142–3151 (2006).
Tu, Y., Wu, S., Shi, X., Chen, K. & Wu, C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47 (2003).
Yamada, K. M., Pankov, R. & Cukierman, E. Dimensions and dynamics in integrin function. Braz. J. Med. Biol. Res. 36, 959–966 (2003).
Morgan, M. R., Humphries, M. J. & Bass, M. D. Synergistic control of cell adhesion by integrins and syndecans. Nature Rev. Mol. Cell Biol. 8, 957–969 (2007).
Acknowledgements
The authors are grateful to K. Yamada for providing the photographs for FIG. 1 and to B. Morgenstern for expert help in preparing this article for publication. The authors' work was partially supported by the Volkswagen Foundation, the National Institutes of Health (NIH; through the NIH Roadmap for Medical Research), the Israel Science Foundation, the Minerva Foundation, the Maurice Janin Fund and the Landesstiftung Baden-Württemberg. B.G. holds the Erwin Neter Professorial Chair in Cell and Tumour Biology. A.D.B. holds the Joseph Moss Professorial Chair in Biomedical Research. J.P.S. is a Weston Visiting Professor at the Weizmann Institute of Science.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
FURTHER INFORMATION
Alexander D. Bershadsky's research
Glossary
- Extracellular matrix
-
(ECM). The complex, multimolecular material that surrounds cells. The ECM comprises a scaffold on which tissues are organized, provides cellular microenvironments and regulates multiple cellular functions.
- Focal adhesion
-
An integrin-mediated cell–substrate adhesion structure that anchors the ends of actin filaments (stress fibres) and mediates strong attachments to substrates. It also functions as an integrin-signalling platform.
- Focal complex
-
A small (1 μm diameter), dot-like adhesion structure that is formed underneath the lamellipodium.
- Lamellipodium
-
A ribbon-like, flat protrusion at the periphery of a moving or spreading cell that is enriched with a branched network of actin filaments.
- Lamella
-
A flat, sheet-like extension that is found at the cell periphery but is more internal than lamellipodia. A fan-shaped lamella is a prominent feature that characterizes the leading edge of a cell that is undergoing locomotion on a flat surface. Actin networks, also containing myosin IIA, are the principal structures in lamellae.
- Filopodium
-
A thin, transient actin protrusion that extends out from the cell surface and is formed by the elongation of bundled actin filaments in its core.
- LIM domain
-
A repeat of ∼60 amino acids that contains Cys and His residues. The LIM domain is thought to be involved in protein–protein interactions.
- Stress fibres
-
Also termed actin-microfilament bundles, these are arrays of parallel filaments that contain filamentous actin and myosin II, and often stretch between cell attachments as if under stress.
Rights and permissions
About this article
Cite this article
Geiger, B., Spatz, J. & Bershadsky, A. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10, 21–33 (2009). https://doi.org/10.1038/nrm2593
Issue Date:
DOI: https://doi.org/10.1038/nrm2593
This article is cited by
-
CD248 promotes migration and metastasis of osteosarcoma through ITGB1-mediated FAK-paxillin pathway activation
BMC Cancer (2023)
-
The spatiotemporal heterogeneity of the biophysical microenvironment during hematopoietic stem cell development: from embryo to adult
Stem Cell Research & Therapy (2023)
-
Allosteric activation of vinculin by talin
Nature Communications (2023)
-
Curved adhesions mediate cell attachment to soft matrix fibres in three dimensions
Nature Cell Biology (2023)
-
Mechanosensitive aquaporins
Biophysical Reviews (2023)