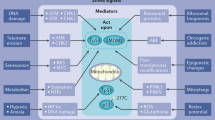
Key Points
-
p53 is an important tumour-suppressor protein that is altered in most cancers.
-
p53 activates various responses, including cell-cycle arrest and apoptosis. Each of these appears to contribute to tumour suppression.
-
p53 responds to both acute stress, such as genotoxic stress and the activation of oncogenes, and constitutive stress induced by factors such as hypoxia or starvation stress. Each of these can contribute to tumour development.
-
In addition to tumour suppression, p53 can also have a role in normal development, during which loss of p53 can be detrimental.
-
The activation of p53 can have undesirable effects that might contribute to diseases such as neurodegenerative syndromes and also to the ageing process in an organism.
Abstract
As a component of the response to acute stress, p53 has a well established role in protecting against cancer development. However, it is now becoming clear that p53 can have a much broader role and can contribute to the development, life expectancy and overall fitness of an organism. Although the function of p53 as a tumour suppressor ensures that we can't live without it, an integrated view of p53 suggests that not all of its functions are conducive to a long and healthy life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Royds, J. A. & Iacopetta, B. p53 and disease: when the guardian angel fails. Cell Death Differ. 13, 1017–1026 (2006).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Donehower, L. A. The p53-deficient mouse: a model for basic and applied cancer studies. Sem. Cancer Biol. 7, 269–278 (1996).
Pietsch, E. C., Humbey, O. & Murphey, M. E. Polymorphisms in the p53 pathway. Oncogene 25, 1602–1611 (2006).
Lapenko, O. & Prives, C. Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 13, 951–961 (2006).
Cawley, S. et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 116, 499–509 (2004).
Wei, C. -L. et al. A global map of p53 transcription-factor binding sites in the human genome. Cell 124, 207–219 (2006).
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006). This paper provides evidence that loss of p53 might contribute to the Warburg effect.
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006).
Crighton, D. et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 14, 121–134 (2006).
Gatz, S. A. & Wiesmuller, L. p53 in recombination and repair. Cell Death Differ. 13, 1003–1016 (2006).
Bensaad, K. & Vousden, K. H. Savior and slayer: the two faces of p53. Nature Med. 11, 1278–1279 (2005).
Roger, L., Gadea, G. & Roux, P. Control of cell migration: a tumour suppressor function for p53? Biol. Cell 98, 141–152 (2006).
Kortleverm, R. M., Higgins, P. J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature Cell Biol. 8, 877–884 (2006).
Toeodoro, J. G., Parker, A. E., Zhu, X. & Green, M. R. p53-mediated inhibtion of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science 313, 968–971 (2006).
Murray-Zmijewski, F., Lane, D. P. & Bourdon, J. C. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 13, 962–972 (2006).
Wang, X. et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 172, 115–125 (2006).
Yee, K. S. & Vousden, K. H. Complicating the complexity of p53. Carcinogenesis 26, 1317–1322 (2005).
Moll, U. M., Wolff, S., Speidel, D. & Deppert, W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 17, 631–636 (2005).
Yu, J. & Zhang, L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell 4, 248–249 (2003).
Strom, E. et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from γ radiation. Nature Chem. Biol. 2, 474–479 (2006).
Chipuk, J. E., Bouchier-Hayes, L., Kuwana, T., Newmeyer, D. D. & Green, D. R. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science 309, 1732–1735 (2005). An elegant study that consolidates the transcriptionally dependent and independent activities of p53 into a single model.
Levine, A. J., Hu, W. & Feng, Z. The p53 pathway: what questions remain to be explored? Cell Death Differ. 13, 1027–1036 (2006).
Brooks, C. L. & Gu, W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307–315 (2006).
Braithwaite, A. W., Del Sal, G. & Lu, X. Some p53-binding proteins that can function as arbiters of life and death. Cell Death Differ. 13, 984–993 (2006).
Harris, S. L. & Levine, A. J. The p53 pathway: positive and negative feedback loops. Oncogene 24, 2899–2908 (2005).
Marine, J. C. et al. Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 13, 927–934 (2006).
Nordstrom, W. & Abrams, J. M. Guardian ancestry: fly p53 and damage-inducible apoptosis. Cell Death Differ. 7, 1035–1038 (2000).
Derry, W. B., Putzke, A. P. & Rothman, J. H. Caenorhabditis elegans p53: role in apoptosis, meiosis and stress resistance. Science 294, 591–595 (2001).
Schumacher, B., Hofmann, K., Boulton, S. & Gartner, A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722–1727 (2001).
Kamijo, T. et al. Loss of the ARF tumor suppressor reverses premature replicative arrest but not radiation hypersensitivity arising from disabled atm function. Cancer Res. 59, 2464–2469 (1999).
Lohrum, M. A., Ludwig, R. L., Kubbutat, M. H. G., Hanlon, M. & Vousden, K. H. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell 3, 577–587 (2003).
Christophorou, M. A., Ringhausen, I., Finch, A. J., Brown Swigart, L. & Evan, G. I. The pathological p53-mediated response to DNA damage is distinct from p53-mediated tumor suppression. Nature 14, 214–217 (2006).
Efeyan, A., Garcia-Cao, I., Herranz, D., Velasco-Miguel, S. & Serrano, M. Policing of oncogene activity by p53. Nature 443, 159 (2006). References 33 and 34 suggest that the response to oncogene activation, but not to DNA damage, is crucial for p53-mediated tumour suppression.
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigensis. Nature 434, 864–870 (2005).
Gorgoulis, V. G. et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913 (2005). Two studies that, in contrast to 33 and 34, show the importance of the DNA-damage signal to tumour suppression.
Lassus, P., Ferlin, M., Piette, J. & Hibner, U. Anti-apoptotic activity of low levels of wild type p53. EMBO J. 15, 4566–4573 (1996).
Jones, R. G. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005).
Stambolsky, P. et al. Regulation of AIF expression by p53. Cell Death Differ. 13, 2140–2149 (2006).
Johnson, T. M., Yu, Z.-X., Ferrans, V. J., Lowenstein, R. A. & Finkel, T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl Acad. Sci. USA 93, 11848–11852 (1996).
Polyak, K., Xia, Y., Zweier, J. L., Kinzler, K. W. & Vogelstein, B. A model for p53-induced apoptosis. Nature 389, 300–305 (1997).
Velasco-Miguel, S. et al. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 18, 127–137 (1999).
Budanov, A. V., Sablina, A. A., Feinstein, E., Koonin, E. V. & Chumakov, P. M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304, 596–600 (2004).
Yoon, K. A., Nakamura, Y. & Arakawa, H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J. Hum. Genet. 49, 134–140 (2004).
Gu, S. et al. Global investigation of p53-induced apoptosis through quantitative proteomic profiling using comparative amino acid-coded tagging. Mol. Cell. Proteomics 3, 998–1008 (2006).
Sablina, A. A. et al. The antioxidant function of the p53 tumor suppressor gene. Nature Med. 11, 1306–1313 (2005). A demonstration of the antioxidant activity of basal levels of p53.
Hemann, M. T. et al. Suppression of tumorigenesis by the p53 target PUMA. Proc. Natl Acad. Sci. USA 101, 9333–9338 (2004).
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004).
Liu, G. et al. Chromosomal stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nature Genet. 36, 63–68 (2004).
Dimri, G. P. What has senescence got to do with cancer? Cancer Cell 7, 505–512 (2005).
Barbieri, C. E. & Pietenpol, J. A. p63 and epithelial biology. Exp. Cell Res. 312, 695–706 (2006).
Flores, E. R. et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 7, 363–373 (2005).
Sutcliffe, J. E. & Brehm, A. Of flies and men; p53, a tumour suppressor. FEBS Lett. 567, 86–91 (2004).
Mills, A. A. p53: link to the past, bridge to the future. Genes Dev. 19, 2091–2099 (2005).
Chio, J. & Donehower, L. A. p53 in embryonic development: maintaining a fine balance. CMLS Cell. Mol. Life Sci. 55, 38–47 (1999).
Armstrong, J. F., Kaufman, M. H., Harrison, D. J. & Clarke, A. R. High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 5, 931–936 (1995).
Sah, V. P. et al. A subset of p53-deficient embyos exhibit exencephaly. Nature Genet. 10, 175–180 (1995).
Hall, P. A. & Lane, D. P. Tumor suppressors: a developing role for p53? Curr. Biol. 7, R144–R147 (1997).
Baatout, S. et al. Developmental abnormalities induced by X-irradiation in p53 deficient mice. In Vivo. 16, 215–221 (2002).
Azuma, M., Toyama, R., Laver, E. & Dawid, I. B. Perturbation of rRNA synthesis in the bap28 mutation leads to apoptosis mediated by p53 in the zebrafish central nervous system. J. Biol. Chem. 281, 13309–13316 (2006).
Plaster, N., Sonntag, C., Busse, C. E. & Hammerschmidt, M. p53 deficiency rescues apoptosis and differentiation of multiple cell types in zebrafish flathead mutants deficient for zygotic DNA polymerase δ1. Cell Death Differ. 13, 223–235 (2006).
Chen, J. et al. Loss of function of def selectively up-regulates Δ113p53 expression to arrest expansion growth of digestive organs in zebrafish. Genes Dev. 19, 2900–2911 (2005).
Campbell, W. A. et al. Zebrafish lacking Alzheimer presenilin enhancer 2 (Pen-2) demonstrate excessive p53-dependent apoptosis and neuronal loss. J. Neurochem. 96, 1423–1440 (2006).
Nicol, C. J., Harrison, M. L., Laposa, R. R., Gimelshtein, I. L. & Well, P. G. A teratologic suppressor role for p53 in benzo(a)pyrene-treated transgenic p53-deficient mice. Nature Genet. 10, 181–187 (1995).
Norimura, T., Nomoto, S., Katsuki, M., Gondo, Y. & Kondo, S. p53-dependent apoptosis suppresses radiation-induced teratogenesis. Nature Med. 2, 577–580 (1996).
Varley, J. M. Germline TP53 mutations and Li–Fraumeni syndrome. Hum. Mutat. 21, 313–320 (2003).
Georgiev, P., Dahm, F., Graf, R. & Clavien, P. A. Blocking the path to death: anti-apoptotic molecules in ischemia/reperfusion injury of the liver. Curr. Pharm. Des. 12, 2911–2921 (2006).
Fiskum, G. et al. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J. Bioenerg. Biomembr. 36, 347–352 (2004).
Dagher, P. C. Apoptosis in ischemic renal injury: roles of GTP depletion and p53. Kidney Int. 66, 506–509 (2004).
Matsusaka, H. et al. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovasc. Res. 70, 457–465 (2006).
Jacobs, W. B., Kaplan, D. R. & Miller, F. D. The p53 family in nervous system development and disease. J. Neurochem. 97, 1571–1584 (2006).
Tyner, S. D. et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002).
Maier, B. et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 18, 306–319 (2004).
van Heemst, D. et al. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp. Gerontol. 40, 11–15 (2005).
Sharpless, N. E. & DePinho, R. A. Telomeres, stem cells, senescence, and cancer. J. Clin. Invest. 113, 160–168 (2004).
Bauer, J. H., Poon, P. C., Glatt-Deeley, H., Abrams, J. M. & Helfand, S. L. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr. Biol. 15, 2063–2068 (2005).
Bauer, J. H. & Helfand, S. L. New tricks of an old molecule: lifespan regulation by p53. Aging Cell 5, 437–440 (2006).
Bourdon, J. C. et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 19, 2122–2137 (2005).
Kondoh, H. et al. Glycolytic enzymes can modulate cellular lifespan. Cancer Res. 65, 177–185 (2005).
Garcia-Cao, I. et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 21, 6225–6235 (2002).
Mendrysa, S. M. et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 20, 16–21 (2006). References 80 and 81 show that increasing the copy number of p53 while maintaining normal control over expression does not result in enhanced ageing.
Mendrysa, S. M. & Perry, M. E. Tumor suppression by p53 without acceletated aging: just enough of a good thing? Cell Cycle 5, 714–717 (2006).
Poyurovsky, M. V. & Prives, C. Unleashing the power of p53: lessons from mice and men. Genes Dev. 20, 125–131 (2006).
Wells, B. S., Yoshida, E. & Johnston, L. A. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr. Biol. 16, 1606–1615 (2006).
Vousden, K. H. & Lu, X. Live or let die: the cell's response to p53. Nature Rev. Cancer 2, 594–604 (2002).
Christophorou, M. A. et al. Temporal diffection of p53 function in vitro and in vivo. Nature Genet. 37, 718–726 (2005).
Soussi, T. & Lozano, G. p53 mutation heterogeneity in cancer. Biochem. Biophys. Res. Com. 331, 834–842 (2005).
Lang, G. A. et al. Gain-of-function of a p53 hot spot mutation in a mouse model of Li–Fraumeni syndrome. Cell 119, 861–872 (2004).
Olive, K. P. et al. Mutant p53 gain-of-function in two mouse models of Li–Fraumeni syndrome. Cell 119, 847–860 (2004). Two sophisticated studies showing the effects of expression of mutant p53 in mouse models.
Irwin, M. S. Family feud in chemosensitivity: p73 and mutant p53. Cell Cycle 3, 319–323 (2004).
Sigal, A. & Rotter, V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 60, 6788–6793 (2000).
Kim, E. & Deppert, W. Transcriptional activities of mutant p53: when mutations are more than a loss. J. Cell Biochem. 93, 878–886 (2004).
Bond, G. L. et al. A single nucleotide polymorphism in the Mdm2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119, 591–602 (2004).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecular antagonists of MDM2. Science 303, 844–848 (2004). One of a number of studies describing small-molecule activators of p53.
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nature Med. 10, 1321–1328 (2004).
Yang, Y. et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7, 547–559 (2005).
Komarov, P. G. et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285, 1733–1737 (1999). The description of a small-molecule inhibitor of p53
Green, D. R. Apoptotic pathways: the roads to ruin. Cell 94, 695–698 (1998).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Cory, S. & Adams, J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nature Rev. Cancer 2, 647–656 (2002).
Jeffers, J. R. et al. Puma is an essential mediator of p53-dependent and-independent apoptotic pathways. Cancer Cell 4, 321–328 (2003).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302, 1036–1038 (2003).
Vousden, K. H. p53 and PUMA: a deadly duo. Science 309, 1685–1686 (2005).
Parks, D. J. et al. Enhanced pharmacokinetic properties of 1,4-benzodiazepine-2,5-dione antagonists of the HDM2–p53 protein–protein interaction through structure-based drug design. Bioog. Med. Chem. Lett. 16, 3310–3314 (2006).
Ding, K. et al. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 127, 10130–10131 (2005).
Lai, Z. et al. Differentiation of Hdm2-mediated p53 ubiquitination and Hdm2 autoubiquitination activity by small molecular weight inhibitors. Proc. Natl Acad. Sci. USA 99, 14734–14739 (2002).
Foster, B. A., Coffey, H. A., Morin, M. J. & Rastinejad, F. Pharmacological rescue of mutant p53 conformation and function. Science 286, 2507–2510 (1999).
Bykov, V. J. N. et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nature Med. 8, 282–288 (2002).
Gudkov, A. V. & Komarova, E. A. Prospective therapeutic applications of p53 inhibitors. Biochem. Biophys. Res. Com. 331, 726–736 (2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- ChIP analysis
-
A chromatin immuno-precipitation technique that identifies proteins that are bound to promoter sequences of genes in the context of endogenous chromatin.
- Glycolysis
-
The metabolic pathway through which glucose is metabolized to provide energy.
- Autophagy
-
A process in which parts of the cytoplasm, including the organelles it contains, are engulfed inside a membranous compartment and targeted to lysosomes for degradation.
- BCL2 family
-
A family of proteins that share structural motifs and have an important role in positively and negatively regulating mitochondrial apoptotic pathways.
- PUMA
-
(p53-upregulated mediator of apoptosis). A protein that belongs to the BCL2 family and that promotes mitochondrial outer membrane permeabilization and apoptosis.
- Ubiquitin ligase
-
An enzyme that can transfer ubiquitin onto a protein, which can target the protein for degradation by the proteasome.
- MDM2
-
A ubiquitin ligase that functions as an important negative regulator of p53. As the product of a p53-inducible gene, MDM2 functions in a negative-feedback loop with p53.
- Warburg effect
-
An increase in aerobic glycolysis that is characteristic of cancer cells.
- TIGAR
-
A gene that encodes a protein that functions to lower intracellular reactive oxygen species levels by enhancing the pentose phosphate pathway.
- Pentose phosphate pathway
-
An alternative pathway for glucose metabolism that generates NADPH, which is needed for the scavenging of reactive oxygen species by reduced glutathione.
- Sestrins
-
A family of proteins that modulate peroxide signalling and regulate intracellular reactive oxygen species levels.
Rights and permissions
About this article
Cite this article
Vousden, K., Lane, D. p53 in health and disease. Nat Rev Mol Cell Biol 8, 275–283 (2007). https://doi.org/10.1038/nrm2147
Issue Date:
DOI: https://doi.org/10.1038/nrm2147
This article is cited by
-
Network pharmacology study to explore the multiple molecular mechanism of SH003 in the treatment of non-small cell lung cancer
BMC Complementary Medicine and Therapies (2024)
-
Combined absence of TRP53 target genes ZMAT3, PUMA and p21 cause a high incidence of cancer in mice
Cell Death & Differentiation (2024)
-
Transcriptional regulation and post-translational modifications in the glycolytic pathway for targeted cancer therapy
Acta Pharmacologica Sinica (2024)
-
Precise pancreatic cancer therapy through targeted degradation of mutant p53 protein by cerium oxide nanoparticles
Journal of Nanobiotechnology (2023)
-
Proteomic Analysis of Protective Effects of Dl-3-n-Butylphthalide against mpp + -Induced Toxicity via downregulating P53 pathway in N2A Cells
Proteome Science (2023)