Key Points
-
T helper 17 (TH17) cells activated by transforming growth factor-β (TGFβ) and interleukin-6 (IL-6) promote mucosal defence, barrier tissue integrity and curtail immunopathogenic responses, whereas IL-23-activated TH17 cells promote chronic tissue inflammation during infection, granuloma formation and autoimmunity.
-
Retinoic acid receptor-related orphan receptor-γt (RORγt) is a TH17 cell-specific master transcription factor. However, it does not act alone, but instead functions as part of a protein complex that regulates TH17 lineage fate. RORγt takes advantage of the open DNA conformation induced by basic leucine zipper transcription factor ATF-like (BATF) and interferon-regulatory factor 4 (IRF4) following T cell receptor stimulation. RORγt also requires the presence of inflammatory cytokine-induced signal transducer and activator of transcription 3 (STAT3) and, together, these transcription factors function as a 'rheostat' that fine-tunes a pre-established TH17 lineage programme.
-
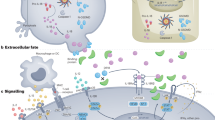
IL-17 signalling is mediated through a distinct cytokine receptor family, which is characterized by a conserved SEF/IL-17R (SEFIR) domain in the cytoplasmic tail. All known IL-17-dependent signalling events occur through ACT1, which controls TNF receptor-associated factor (TRAF)-dependent activation of downstream signalling components (for example, mitogen-activated protein kinases) and transcription factors (for example, nuclear factor-κB (NF-κB) and CCAAT/enhancer-binding proteins (C/EBPs) and mRNA stability.
-
IL-17 signal transduction is restricted by multiple downstream events, involving inhibitory transcription factors, ubiquitylation/deubiquitylation of signalling intermediates, microRNA regulation and control of target mRNA stability.
-
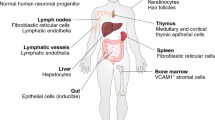
In vivo, IL-17 is an essential regulator of immunity to fungi, particularly the commensal fungus Candida albicans. Humans with congenic or acquired blockade of the IL-17 signalling pathway are particularly susceptible to chronic mucosal candidiasis.
-
The therapeutic strategy of targeting IL-17 and IL-23 shows encouraging results for psoriasis, Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis.
Abstract
Following the discovery of T helper 17 (TH17) cells, the past decade has witnessed a major revision of the TH subset paradigm and substantial progress has been made in deciphering the molecular mechanisms of T cell lineage commitment and function. In this Review, we focus on the recent advances that have been made regarding the transcriptional control of TH17 cell plasticity and stability, as well as the effector functions of TH17 cells, and we highlight the mechanisms of IL-17 signalling in mesenchymal and barrier epithelial tissues. We also discuss the emerging clinical data showing that IL-17-specific and IL-23-specific antibody treatments are remarkably effective for treating many immune-mediated inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003). This study shows that IL-23 controls a key checkpoint for the induction of autoimmune inflammation.
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Kastelein, R. A., Hunter, C. A. & Cua, D. J. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25, 221–242 (2007).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005). This is the first study to suggest that IL-17-producing cells are crucial mediators of autoimmunity, and it led to the proposal of the T H 17 hypothesis.
Murphy, C. A. et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005). This study coined the term 'T H 17' cells to describe a unique lineage that is STAT3 dependent, rather than STAT4- and STAT6-independent.
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 6, 1133–1141 (2005). This is one of the first papers suggesting the existence of IL-17-producing inflammatory T cells.
Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A. & Coffman, R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 (1986). This is the landmark paper proposing the T H 1–T H 2 hypothesis.
Mosmann, T. R. & Coffman, R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Parham, C. et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168, 5699–5708 (2002).
Weaver, C. T., Hatton, R. D., Mangan, P. R. & Harrington, L. E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 (2007).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006). This study describes the discovery of a novel transcriptional regulator that controls Il17a expression and provides the definitive proof that T H 17 cells belong to a new lineage of CD4+ T H cells.
Cua, D. J. & Tato, C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nature Rev. Immunol. 10, 479–489 (2010).
Annunziato, F., Cosmi, L., Liotta, F., Maggi, E. & Romagnani, S. Type 17 T helper cells—origins, features and possible roles in rheumatic disease. Nature Rev. Rheumatol. 5, 325–331 (2009).
Zuniga, L. A., Jain, R., Haines, C. & Cua, D. J. Th17 cell development: from the cradle to the grave. Immunol. Rev. 252, 78–88 (2013).
Kim, J. S. et al. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nature Immunol. 14, 611–618 (2013).
Marks, B. R. et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nature Immunol. 10, 1125–1132 (2009).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
El-Behi, M. et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature Immunol. 12, 568–575 (2011).
Codarri, L. et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature Immunol. 12, 560–567 (2011).
McGeachy, M. J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nature Immunol. 8, 1390–1397 (2007).
Hirota, K. et al. Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nature Immunol. 14, 372–379 (2013).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Chackerian, A. A. et al. Neutralization or absence of the interleukin-23 pathway does not compromise immunity to mycobacterial infection. Infect. Immun. 74, 6092–6099 (2006).
Chen, Y. et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116, 1317–1326 (2006).
Lieberman, L. A. et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol. 173, 1887–1893 (2004).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006). References 27–29 show the importance of TGFβ plus IL-6 in the lineage specification of T H 17 cells.
Yang, X. O. et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363 (2007).
Durant, L. et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32, 605–615 (2010).
Samoilova, E. B., Horton, J. L., Hilliard, B., Liu, T. S. & Chen, Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 161, 6480–6486 (1998).
Alonzi, T. et al. Interleukin 6 is required for the development of collagen-induced arthritis. J. Exp. Med. 187, 461–468 (1998).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Spolski, R. & Leonard, W. J. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr. Opin. Immunol. 20, 295–301 (2008).
Sutton, C., Brereton, C., Keogh, B., Mills, K. H. & Lavelle, E. C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691 (2006).
Chung, Y. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30, 576–587 (2009).
Gulen, M. F. et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity 32, 54–66 (2010). This paper shows that IL-1 is a key factor that provides a key competitive advantage for in vivo T H 17 cell expansion and survival during inflammatory conditions by inducing catabolic energy pathways.
Veldhoen, M., Hocking, R. J., Flavell, R. A. & Stockinger, B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nature Immunol. 7, 1151–1156 (2006).
Li, M. O., Wan, Y. Y. & Flavell, R. A. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26, 579–591 (2007).
Gutcher, I. et al. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity 34, 396–408.
Das, J. et al. Transforming growth factor β is dispensable for the molecular orchestration of Th17 cell differentiation. J. Exp. Med. 206, 2407–2416 (2009).
Volpe, E. et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nature Immunol. 9, 650–657 (2008).
Manel, N., Unutmaz, D. & Littman, D. R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunol. 9, 641–649 (2008).
Acosta-Rodriguez, E. V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nature Immunol. 8, 942–949 (2007).
Wilson, N. J. et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunol. 8, 950–957 (2007).
Chen, Z., Tato, C. M., Muul, L., Laurence, A. & O'Shea, J. J. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 56, 2936–2946 (2007).
Yang, Y. et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 206, 1549–1564 (2009).
Jager, A., Dardalhon, V., Sobel, R. A., Bettelli, E. & Kuchroo, V. K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 183, 7169–7177 (2009).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nature Immunol. 10, 314–324 (2009). This paper describes the crucial roles of IL-23 for the in vivo expansion and function of T H 17 cells during inflammation.
Haines, C. J. et al. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep. 3, 1378–1388 (2013).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Kebir, H. et al. Preferential recruitment of interferon-γ-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 66, 390–402 (2009).
Hirota, K. et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature Immunol. 12, 255–263 (2011). This elegant study used an Il17a fate-mapping strategy to demonstrate the existence of “ex-T H 17” cells driving autoimmune pathology.
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nature Immunol. 13, 991–999 (2012).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Liu, Y. et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 4, e1000041 (2008).
Reveille, J. D. et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nature Genet. 42, 123–127 (2010).
Lock, C. et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature Med. 8, 500–508 (2002).
Burton, P. R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nature Genet. 39, 1329–1337 (2007).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Diveu, C. et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 182, 5748–5756 (2009). This study shows that IL-27 is an inhibitor of the T H 17 immune pathway and explores the mechanisms underlying IL-27-mediated regulation of inflammation.
Laurence, A. et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007).
El-behi, M. et al. Differential effect of IL-27 on developing versus committed Th17 cells. J. Immunol. 183, 4957–4967 (2009).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nature Immunol. 11, 854–861 (2010).
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Minegishi, Y. et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448, 1058–1062 (2007).
Ise, W. et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature Immunol. 12, 536–543 (2011).
Schraml, B. U. et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature 460, 405–409 (2009).
Lohoff, M. et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc. Natl Acad. Sci. USA 99, 11808–11812 (2002).
Brustle, A. et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nature Immunol. 8, 958–966 (2007).
Li, P. et al. BATF–JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012). This study argues against the hypothesis that RORγt is the only factor that regulates the specification of the T H 17 lineage.
Oestreich, K. J. & Weinmann, A. S. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nature Rev. Immunol. 12, 799–804 (2012).
Vahedi, G. et al. STATs shape the active enhancer landscape of T cell populations. Cell 151, 981–993 (2012).
Samstein, R. M. et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell 151, 153–166 (2012).
Dang, E. V. et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Chen, Z. et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 (2013).
van Loosdregt, J. et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 39, 259–271 (2013).
Yang, X. O. et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29, 44–56 (2008).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Lee, Y. K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Morrison, P. J. et al. Th17-cell plasticity in Helicobacter hepaticus-induced intestinal inflammation. Mucosal Immunol. 6, 1143–1156 (2013).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Onishi, R. & Gaffen, S. L. IL-17 and its target genes: mechanisms of IL-17 function in disease. Immunology 129, 311–321 (2010).
Yao, Z. et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3, 811–821 (1995).
Ishigame, H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009).
Novatchkova, M., Leibbrandt, A., Werzowa, J., Neubuser, A. & Eisenhaber, F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 28, 226–229 (2003).
Rickel, E. A. et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J. Immunol. 181, 4299–4310 (2008).
Bordon, Y. Cytokines: IL-17C joins the family firm. Nature Rev. Immunol. 11, 805 (2011).
Qian, Y. et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature Immunol. 8, 247–256 (2007).
Chang, S. H., Park, H. & Dong, C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 281, 35603–35607 (2006).
Li, X. Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine 41, 105–113 (2008).
Liu, C. et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2, ra63 (2009).
Schwandner, R., Yamaguchi, K. & Cao, Z. Requirement of tumor necrosis factor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 191, 1233–1239 (2000).
Sun, D. et al. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nature Immunol. 12, 853–860 (2011).
Herjan, T. et al. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J. Immunol. 191, 640–649 (2013).
Bulek, K. et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nature Immunol. 12, 844–852 (2011).
Qu, F. et al. TRAF6-dependent Act1 phosphorylation by the IκB kinase-related kinases suppresses interleukin-17-induced NF-κB activation. Mol. Cell. Biol. 32, 3925–3937 (2012).
Wang, C. et al. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nature Immunol. 14, 72–81 (2013).
Sonder, S. U. et al. IL-17-induced NF-κB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J. Biol. Chem. 286, 12881–12890 (2011).
Huffmeier, U. et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nature Genet. 42, 996–999 (2010).
Shen, F., Ruddy, M. J., Plamondon, P. & Gaffen, S. L. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-α-induced genes in bone cells. J. Leukoc. Biol. 77, 388–399 (2005).
Acosta-Rodriguez, E. V. et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunol. 8, 639–646 (2007).
Yang, D. et al. β-Defensins: Linking innate immunity and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528 (1999).
Goetz, D. H. et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 10, 1033–1043 (2002).
Ruddy, M. J. et al. Functional cooperation between interleukin-17 and tumor necrosis factor-a is mediated by CCAAT/enhancer binding protein family members. J. Biol. Chem. 279, 2559–2567 (2004).
Shen, F., Hu, Z., Goswami, J. & Gaffen, S. L. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 281, 24138–24148 (2006).
Patel, D. N. et al. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-κB and C/EBPβ activation. J. Biol. Chem. 282, 27229–27238 (2007).
Ramji, D. P. & Foka, P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 365, 561–575 (2002).
Shen, F. et al. IL-17 receptor signaling inhibits C/EBPβ by sequential phosphorylation of the regulatory 2 domain. Sci. Signal. 2, ra8 (2009).
Zrioual, S. et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J. Immunol. 182, 3112–3120 (2009).
Shen, F. & Gaffen, S. L. Structure–function relationships in the IL-17 receptor: Implications for signal transduction and therapy. Cytokine 41, 92–104 (2008).
Karlsen, J. R., Borregaard, N. & Cowland, J. B. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-α is controlled by IκB-ζ but neither by C/EBP-β nor C/EBP-δ. J. Biol. Chem. 285, 14088–14100 (2010).
Zhong, B. et al. Negative regulation of IL-17-mediated signaling and inflammation by the ubiquitin-specific protease USP25. Nature Immunol. 13, 1110–1117 (2012).
Garg, A. V., Ahmed, M., Vallejo, A. N., Ma, A. & Gaffen, S. L. The deubiquitinase A20 mediates feedback inhibition of interleukin-17 receptor signaling. Sci. Signal. 6, ra44 (2013). References 118 and 119 show that IL-17R signalling is restrained by multiple deubiquitylating enzymes that target TRAF6.
Ma, A. & Malynn, B. A. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nature Rev. Immunol. 12, 774–785 (2012).
Zhu, S. et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J. Exp. Med. 207, 2647–2662 (2010).
Maitra, A. et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc. Natl Acad. Sci, USA 104, 7506–7511 (2007).
Shembade, N. & Harhaj, E. W. Regulation of NF-κB signaling by the A20 deubiquitinase. Cell. Mol. Immunol. 9, 123–130 (2012).
Iha, H. et al. Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-κB activation. EMBO J. 27, 629–641 (2008).
Shembade, N. et al. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nature Immunol. 9, 254–262 (2008).
Garg, A. V. & Gaffen, S. L. IL-17 signaling and A20: a balancing act. Cell Cycle 12, 3459–3460 (2013).
Ho, A. W. et al. The anaphase-promoting complex protein 5 (AnapC5) associates with A20 and inhibits IL-17-mediated signal transduction. PLoS ONE 8, e70168 (2013).
Shi, P. et al. Persistent stimulation with interleukin-17 desensitizes cells through SCFβ-TrCP-mediated degradation of Act1. Sci. Signal. 4, ra73 (2011).
Xie, P. TRAF molecules in cell signaling and in human diseases. J. Mol. Signal 8, 1–31 (2013).
Zepp, J. A. et al. Cutting edge: TNF receptor-associated factor 4 restricts IL-17-mediated pathology and signaling processes. J. Immunol. 189, 33–37 (2012).
O'Connell, R. M. et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33, 607–619 (2010).
Yao, R. et al. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS ONE 7, e46082 (2012).
Murugaiyan, G., Beynon, V., Mittal, A., Joller, N. & Weiner, H. L. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 187, 2213–2221 (2011).
Zhu, S. et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nature Med. 18, 1077–1086 (2012). This is the first identification of an miRNA feedback loop in the IL-17R signalling pathway.
Milner, J. D. & Holland, S. M. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nature Rev. Immunol. 13, 635–648 (2013).
von Bernuth, H. et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321, 691–696 (2008).
Ma, C. S. et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205, 1551–1557 (2008).
Glocker, E. O. et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735 (2009).
Liu, L. et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208, 1635–1648 (2011).
van de Veerdonk, F. L. et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 365, 54–61 (2011).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010).
Kisand, K. et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 207, 299–308 (2010).
Puel, A. et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68 (2011).
Boisson, B. et al. A biallelic ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 39, 676–686 (2013). References 143 and 144 directly link mucosal C. albicans infections with the IL-17R-mediated signalling axis.
Hernández-Santos, N. & Gaffen, S. L. Th17 cells in immunity to Candida albicans. Cell Host Microbe 11, 425–435 (2012).
Stark, M. A. et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 (2005).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nature Med. 18, 1069–1076 (2012).
Chan, J. R. et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 203, 2577–2587 (2006).
Tonel, G. et al. Cutting edge: A critical functional role for IL-23 in psoriasis. J. Immunol. 185, 5688–5691 (2010).
Perera, G. K., Di Meglio, P. & Nestle, F. O. Psoriasis. Annu. Rev. Pathol. 7, 385–422 (2012).
Villanova, F. et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Invest. Dermatol. 134, 984–991 (2014).
Guttman-Yassky, E. et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 181, 7420–7427 (2008).
Rudwaleit, M. et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 70, 25–31 (2011).
van Echteld, I. et al. Identification of the most common problems by patients with ankylosing spondylitis using the international classification of functioning, disability and health. J. Rheumatol 33, 2475–2483 (2006).
Mielants, H. et al. The evolution of spondyloarthropathies in relation to gut histology. II. Histological aspects. J. Rheumatol 22, 2273–2278 (1995).
Cotterill, L. et al. Replication and meta-analysis of 13,000 cases defines the risk for interleukin-23 receptor and autophagy-related 16-like 1 variants in Crohn's disease. Can. J. Gastroenterol. 24, 297–302 (2010).
Lesage, S. et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am. J. Hum. Genet. 70, 845–857 (2002).
Fujino, S. et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70 (2003).
Dige, A. et al. Increased levels of circulating Th17 cells in quiescent versus active Crohn's disease. J. Crohns Colitis 7, 248–255 (2013).
Leonardi, C. et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 366, 1190–1199 (2012).
Rich, P. et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br. J. Dermatol. 168, 402–411 (2013).
Papp, K. A. et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 366, 1181–1189 (2012). References 160–162 report clinical trials that reveal the remarkable efficacy of IL-17- or IL-17RA-specific antibody therapy for the treatment of psoriasis.
Papp, K. A. et al. Dose-dependent improvement in chronic plaque psoriasis following treatment with anti-IL-23p19 humanized monoclonal antibody (MK-3222). Late-breaking Research Symposium. 71st Annual Meeting of the American Academy of Dermatology (2013).
Chiricozzi, A. & Krueger, J. G. IL-17 targeted therapies for psoriasis. Expert Opin. Investig. Drugs 22, 993–1005 (2013).
Sandborn, W. J. et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N. Engl. J. Med. 367, 1519–1528 (2012).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Targan, S. R. et al. A randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and efficacy of AMG 827 in subjects with moderate to severe Crohn's disease. 143, e26 (2012).
Ogawa, A., Andoh, A., Araki, Y., Bamba, T. & Fujiyama, Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110, 55–62 (2004).
Patel, D. D., Lee, D. M., Kolbinger, F. & Antoni, C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 72, Suppl. 2, iii116–iii123 (2013).
Garber, K. Anti-IL-17 mAbs herald new options in psoriasis. Nature Biotech. 30, 475–477 (2012).
Nakamura, R. et al. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by γδ T cells. J. Immunol. 181, 2071–2075 (2008).
Ishizaki, M. et al. Tyk2 is a therapeutic target for psoriasis-like skin inflammation. Int. Immunol. 26, 257–267 (2013).
Solt, L. A. et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 (2011).
Huh, J. R. et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472, 486–490 (2011).
Xiao, S. et al. Small-molecule RORγt antagonists inhibit T helper 17 Cell transcriptional network by divergent mechanisms. Immunity 40, 477–489 (2014).
Lee, J. S. & Cua, D. J. The emerging landscape of RORγt biology. Immunity 40, 451–452 (2014).
Stritesky, G. L., Jameson, S. C. & Hogquist, K. A. Selection of self-reactive T cells in the thymus. Annu. Rev. Immunol. 30, 95–114 (2012).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 (2005).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nature Rev. Immunol. 13, 145–149 (2013).
Plantinga, T. S. et al. Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 49, 724–732 (2009).
Minegishi, Y. et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25, 745–755 (2006).
Prando, C. et al. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine 92, 109–122 (2013).
de Beaucoudrey, L. et al. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine 89, 381–402 (2010).
Ouederni, M. et al. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor β1 deficiency. Clin. Infect. Dis. 58, 204–213 (2014).
Acknowledgements
S.L.G. was supported by US National Institutes of Health (NIH) grants AI107825 and DE022550. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.L.G. has received research grants from Novartis and Janssen, and has consulted for and received travel reimbursements and/or honouraria from Novartis, Amgen, Pfizer, Eli Lilly and Janssen. R.J. and D.J.C. are employed by Merck and Co. A.V.G. declares no competing interests.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Summary of phase II clinical trials of clinical testing of anti-interleukin-23 (IL-23) and anti-IL-17 treatment for psoriasis (PDF 616 kb)
Glossary
- Crohn's disease
-
A type of inflammatory bowel disease that affects any part of the gastrointestinal tract from the mouth to the anus. Symptoms include abdominal pain, bloody diarrhoea, fever and weight loss. Other complications may occur outside the gastrointestinal tract and include anaemia, skin rashes, arthritis, inflammation of the eye and an increased risk of bowel cancer.
- Chromatin immunoprecipitation followed by sequencing
-
(ChIP–seq). A technique used to analyse the interactions between transcription factors and their target DNA. ChIP–seq combines chromatin immunoprecipitation (ChIP) with massively parallel DNA sequencing to identify the specific sequences bound by regulatory proteins.
- Hyper-IgE syndrome
-
An inherited immune deficiency that is usually caused by mutations in signal transducer and activator of transcription 3 (STAT3) and is associated with reduced T helper 17 (TH17) cell frequency. The disease is characterized by elevated levels of serum IgE, eosinophilia, 'cold' staphylococcal abscesses, eczema, pulmonary infections and chronic mucocutaneous candidiasis.
- Antimicrobial peptides
-
Short peptides (typically 12–50 amino acids) with bactericidal and fungicidal activities. Some may also exhibit chemotactic activities.
- A20
-
The product of the tumour necrosis factor-α-induced protein 3 (TNFAIP3) gene. This is a zinc finger-containing protein with E3 ligase and deubiquitylase activity that downregulates signalling by multiple inflammatory effectors.
- microRNAs
-
(miRNAs). Single-stranded RNA molecules of approximately 21–23 nucleotides in length that regulate gene expression.
- Autoimmune polyendocrinopathy syndrome 1
-
(APS1; also known as autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) syndrome). An inherited autoimmune disorder that affects multiple endocrine tissues and is caused by mutations in the autoimmune regulator (AIRE) gene. The disease is associated with a high incidence of mucocutaneous candidiasis, which is thought to be owing to neutralizing autoantibodies against interleukin-17A (IL-17A), IL-17F and/or IL-22.
Rights and permissions
About this article
Cite this article
Gaffen, S., Jain, R., Garg, A. et al. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14, 585–600 (2014). https://doi.org/10.1038/nri3707
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3707
This article is cited by
-
Cytokines inhibitory mechanism of Prunus domestica L. (Plum) peptides as potential immunomodulators against systemic lupus erythematosus: an in-silico screening
In Silico Pharmacology (2024)
-
Advancements in Bullous Pemphigoid Treatment: A Comprehensive Pipeline Update
American Journal of Clinical Dermatology (2024)
-
Dual-specificity phosphatases 22-deficient T cells contribute to the pathogenesis of ankylosing spondylitis
BMC Medicine (2023)
-
Identification immune response genes in psoriasis after treatment with secukinumab
BMC Medical Genomics (2023)
-
Gateway reflexes describe novel neuro-immune communications that establish immune cell gateways at specific vessels
Bioelectronic Medicine (2023)