Key Points
-
The use of alternative donors, including umbilical cord blood and haploidentical donors, has enabled more patients to undergo haematopoietic cell transplantation (HCT). Recent studies also provide potential solutions for problems associated with the use of alternative donors, such as low stem cell content, increased graft rejection and graft-versus-host-disease (GVHD), and slow immune reconstitution.
-
Reduced intensity conditioning allows more patients to be eligible for HCT. In addition to allowing engraftment, some conditioning regimens may have the ability to reduce the incidence of GVHD while preserving graft-versus-tumour (GVT) effects.
-
Multiple approaches have been proposed to enhance immune reconstitution following HCT. Some of these approaches are currently being evaluated in clinical trials.
-
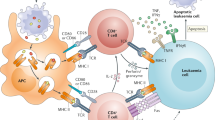
Alloresponses can directly mediate antitumour effects or indirectly promote antitumour immunity by generating a pro-inflammatory environment. In addition, autologous HCT can be used as haematopoietic rescue for patients receiving intensive conditioning to enhance the efficacy of antitumour effects mediated by tumour-specific T cells.
-
Allogeneic HCT has been used to induce tolerance for allogeneic kidney grafts in patients. Induction of mixed chimerism by xenogeneic HCT achieves T cell, B cell and natural killer cell tolerance to xenografts.
-
Both autologous and allogeneic HCT have been used to treat severe autoimmune diseases. Efforts are needed to elucidate the mechanisms of efficacy of these treatments.
Abstract
Haematopoietic cell transplantation (HCT) is the most widely used form of cellular therapy. It is the only known cure for some haematological malignancies and has recently been used in additional clinical settings, such as allograft tolerance induction and treatment of autoimmune diseases. Recent advances have enabled HCT in a wider range of patients with improved outcomes. This Review summarizes the latest developments in this therapy, focusing on issues that will affect future advancement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gyurkocza, B., Rezvani, A. & Storb, R. F. Allogeneic hematopoietic cell transplantation: the state of the art. Expert. Rev. Hematol. 3, 285–299 (2010).
Blazar, B. R., Murphy, W. J. & Abedi, M. Advances in graft-versus-host disease biology and therapy. Nature Rev. Immunol. 12, 443–458 (2012).
Barker, J. N. et al. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood 97, 2957–2961 (2001).
Rocha, V. et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. N. Engl. J. Med. 342, 1846–1854 (2000).
Chen, L., Cohen, A. C. & Lewis, D. B. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol. Blood Marrow Transplant. 12, 160–171 (2006).
Lee, C. C., Lin, S. J., Cheng, P. J. & Kuo, M. L. The regulatory function of umbilical cord blood CD4+ CD25+ T cells stimulated with anti-CD3/anti-CD28 and exogenous interleukin (IL)-2 or IL-15. Pediatr. Allergy Immunol. 20, 624–632 (2009).
Wing, K., Ekmark, A., Karlsson, H., Rudin, A. & Suri-Payer, E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology 106, 190–199 (2002).
Encabo, A., Solves, P., Carbonell-Uberos, F. & Minana, M. D. The functional immaturity of dendritic cells can be relevant to increased tolerance associated with cord blood transplantation. Transfusion 47, 272–279 (2007).
Naderi, N., Pourfathollah, A. A., Alimoghaddam, K. & Moazzeni, S. M. Cord blood dendritic cells prevent the differentiation of naive T-helper cells towards Th1 irrespective of their subtype. Clin. Exp. Med. 9, 29–36 (2009).
Barker, J. N. et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 105, 1343–1347 (2005).
Jacobson, C. A. et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol. Blood Marrow Transplant. 18, 565–574 (2012).
Verneris, M. R. et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 114, 4293–4299 (2009).
Delaney, C. et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nature Med. 16, 232–236 (2010).
Robinson, S. N. et al. Mesenchymal stem cells in ex vivo cord blood expansion. Best Pract. Res. Clin. Haematol. 24, 83–92 (2011).
Bernardo, M. E. et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 46, 200–207 (2011).
MacMillan, M. L., Blazar, B. R., DeFor, T. E. & Wagner, J. E. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant. 43, 447–454 (2008).
Castello, S. et al. Intra-bone marrow injection of bone marrow and cord blood cells: an alternative way of transplantation associated with a higher seeding efficiency. Exp. Hematol. 32, 782–787 (2004).
Kushida, T. et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood 97, 3292–3299 (2001).
Frassoni, F. et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 9, 831–839 (2008).
Frassoni, F. et al. The intra-bone marrow injection of cord blood cells extends the possibility of transplantation to the majority of patients with malignant hematopoietic diseases. Best Pract. Res. Clin. Haematol. 23, 237–244 (2010).
Bautista, G. et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 43, 365–373 (2008).
Liu, H. et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood 118, 6438–6445 (2011).
Sebrango, A. et al. Haematopoietic transplants combining a single unrelated cord blood unit and mobilized haematopoietic stem cells from an adult HLA-mismatched third party donor. Comparable results to transplants from HLA-identical related donors in adults with acute leukaemia and myelodysplastic syndromes. Best Pract. Res. Clin. Haematol. 23, 259–274 (2010).
Lang, P. et al. Transplantation of a combination of CD133+ and CD34+ selected progenitor cells from alternative donors. Br. J. Haematol. 124, 72–79 (2004).
Bethge, W. A. et al. Haploidentical allogeneic hematopoietic cell transplantation in adults with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp. Hematol. 34, 1746–1752 (2006).
Bethge, W. A. et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol. Dis. 40, 13–19 (2008).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002).
Ruggeri, L. et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood 110, 433–440 (2007).
van Rood, J. J. et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood 99, 1572–1577 (2002).
van Rood, J. J. et al. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc. Natl Acad. Sci. USA 106, 19952–19957 (2009).
Akiyama, Y. et al. Transplantation tolerance to a single noninherited MHC class I maternal alloantigen studied in a TCR-transgenic mouse model. J. Immunol. 186, 1442–1449 (2011).
Ball, L. M. et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 110, 2764–2767 (2007).
Nasef, A. et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 84, 231–237 (2007).
Le Blanc, K. et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 (2010).
Lucchini, G. et al. Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol. Blood Marrow Transplant. 16, 1293–1301 (2010).
Ringden, O. & Le Blanc, K. Mesenchymal stem cells for treatment of acute and chronic graft-versus-host disease, tissue toxicity and hemorrhages. Best Pract. Res. Clin. Haematol. 24, 65–72 (2011).
Hoffmann, P., Ermann, J., Edinger, M., Fathman, C. G. & Strober, S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196, 389–399 (2002).
Di Ianni, M. et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011).
Hill, G. R. et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 90, 3204–3213 (1997).
Kasamon, Y. L. et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol. Blood Marrow Transplant. 16, 482–489 (2010).
Ogawa, H. et al. Unmanipulated HLA 2–3 antigen-mismatched (haploidentical) stem cell transplantation using nonmyeloablative conditioning. Biol. Blood Marrow Transplant. 12, 1073–1084 (2006).
Spitzer, T. R. et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation 75, 1748–1751 (2003).
Brunstein, C. G. et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 118, 282–288 (2011).
Pillai, A. B., George, T. I., Dutt, S. & Strober, S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood 113, 4458–4467 (2009).
Lan, F. et al. Predominance of NK1.1+TCR αβ+ or DX5+TCR αβ+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J. Immunol. 167, 2087–2096 (2001).
Lan, F., Zeng, D., Higuchi, M., Higgins, J. P. & Strober, S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol. Blood Marrow Transplant. 9, 355–363 (2003).
Kohrt, H. E. et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood 114, 1099–1109 (2009).
Lowsky, R. et al. Protective conditioning for acute graft-versus-host disease. N. Engl. J. Med. 353, 1321–1331 (2005). The non-myeloablative conditioning regimen used in references 47 and 48 in combination with HLA-identical transplantation is associated with very low rates of acute GVHD.
Spitzer, T. R. et al. Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol. Blood Marrow Transplant. 6, 309–320 (2000).
Shaffer, J. et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp. Hematol. 35, 1140–1152 (2007).
Luznik, L. et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 14, 641–650 (2008).
Munchel, A. et al. Nonmyeloablative, HLA-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr. Rep. 3, 12–14 (2011).
Sathe, A., Ortega, S. B., Mundy, D. I., Collins, R. H. & Karandikar, N. J. In vitro methotrexate as a practical approach to selective allodepletion. Biol. Blood Marrow Transplant. 13, 644–654 (2007).
Watson, D. Tolerance induction by removal of alloreactive T cells: in-vivo and pruning strategies. Curr. Opin. Organ Transplant. 14, 357–363 (2009).
Godfrey, W. R., Krampf, M. R., Taylor, P. A. & Blazar, B. R. Ex vivo depletion of alloreactive cells based on CFSE dye dilution, activation antigen selection, and dendritic cell stimulation. Blood 103, 1158–1165 (2004).
Amrolia, P. J. et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood 108, 1797–1808 (2006).
McIver, Z. A. et al. Immune reconstitution in recipients of photodepleted HLA-identical sibling donor stem cell transplantations: T cell subset frequencies predict outcome. Biol. Blood Marrow Transplant. 17, 1846–1854 (2011).
Solomon, S. R. et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood 106, 1123–1129 (2005).
Samarasinghe, S. et al. Functional characterization of alloreactive T cells identifies CD25 and CD71 as optimal targets for a clinically applicable allodepletion strategy. Blood 115, 396–407 (2010).
Amrolia, P. J. et al. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood 102, 2292–2299 (2003).
Szmania, S. et al. Isolation and expansion of cytomegalovirus-specific cytotoxic T lymphocytes to clinical scale from a single blood draw using dendritic cells and HLA-tetramers. Blood 98, 505–512 (2001).
Schmitt, A. et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion 51, 591–599 (2011).
Feuchtinger, T. et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br. J. Haematol. 134, 64–76 (2006).
Heslop, H. E. et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115, 925–935 (2010).
Khanna, N. et al. Generation of a multipathogen-specific T-cell product for adoptive immunotherapy based on activation-dependent expression of CD154. Blood 118, 1121–1131 (2011).
Barker, J. N. et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 116, 5045–5049 (2010).
Haque, T. et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 110, 1123–1131 (2007).
Dunon, D., Allioli, N., Vainio, O., Ody, C. & Imhof, B. A. Quantification of T-cell progenitors during ontogeny: thymus colonization depends on blood delivery of progenitors. Blood 93, 2234–2243 (1999).
Zlotoff, D. A. et al. Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation. Blood 118, 1962–1970 (2011).
Zakrzewski, J. L. et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nature Med. 12, 1039–1047 (2006).
Dallas, M. H., Varnum-Finney, B., Martin, P. J. & Bernstein, I. D. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand δ1. Blood 109, 3579–3587 (2007).
Zakrzewski, J. L. et al. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nature Biotech. 26, 453–461 (2008).
Eyrich, M. et al. Pre-differentiated human committed T-lymphoid progenitors promote peripheral T-cell re-constitution after stem cell transplantation in immunodeficient mice. Eur. J. Immunol. 41, 3596–3603 (2011).
Ciceri, F. et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 10, 489–500 (2009).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011). These two clinical trials (references 74 and 75) demonstrate that the transfer of suicide gene-transduced donor T cells to enhance immune reconstitution is safe because GVHD can be effectively controlled.
Hollander, G. A., Krenger, W. & Blazar, B. R. Emerging strategies to boost thymic function. Curr. Opin. Pharmacol. 10, 443–453 (2010).
Seggewiss, R. & Einsele, H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood 115, 3861–3868 (2010).
Levine, J. E., Blazar, B. R., DeFor, T., Ferrara, J. L. M. & Weisdorf, D. J. Long-term follow-up of a phase I/II randomized, placebo-controlled trial of palifermin to prevent graft-versus-host disease (GVHD) after related donor allogeneic hematopoietic cell transplantation (HCT). Biol. Blood Marrow Transplant. 14, 1017–1021 (2008).
Sutherland, J. S. et al. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin. Cancer. Res. 14, 1138–1149 (2008).
Kelly, R. M. et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood 111, 5734–5744 (2008).
Colvin, G. A. et al. Nonengraftment haploidentical cellular immunotherapy for refractory malignancies: tumor responses without chimerism. Biol. Blood Marrow Transplant. 15, 421–431 (2009).
Dey, B. R. et al. Anti-tumour response despite loss of donor chimaerism in patients treated with non-myeloablative conditioning and allogeneic stem cell transplantation. Br. J. Haematol. 128, 351–359 (2005).
Guo, M. et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood 117, 936–941 (2011).
Spitzer, T. R. et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation 91, 672–676 (2011). References 81–84 collectively show that rejection of allogeneic haematopoietic cells can be associated with tumour responses without GVHD.
Rubio, M. T. et al. Antitumor effect of donor marrow graft rejection induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: critical role for recipient-derived IFN-γ. Blood 102, 2300–2307 (2003).
Saito, T. I., Rubio, M. T. & Sykes, M. Clinical relevance of recipient leukocyte infusion as antitumor therapy following nonmyeloablative allogeneic hematopoietic cell transplantation. Exp. Hematol. 34, 1270–1276 (2006).
Saito, T. I., Li, H. W. & Sykes, M. Invariant NKT cells are required for antitumor responses induced by host-versus-graft responses. J. Immunol. 185, 2099–2105 (2010).
Rubio, M. T., Zhao, G., Buchli, J., Chittenden, M. & Sykes, M. Role of indirect allo- and autoreactivity in anti-tumor responses induced by recipient leukocyte infusions (RLI) in mixed chimeras prepared with nonmyeloablative conditioning. Clin. Immunol. 120, 33–44 (2006).
Rubio, M. T. et al. Mechanisms of the antitumor responses and host-versus-graft reactions induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: a critical role for recipient CD4+ T cells and recipient leukocyte infusion-derived IFN-γ-producing CD8+ T cells. J. Immunol. 175, 665–676 (2005).
Stelljes, M. et al. Graft-versus-host disease after allogeneic hematopoietic stem cell transplantation induces a CD8+ T cell-mediated graft-versus-tumor effect that is independent of the recognition of alloantigenic tumor targets. Blood 104, 1210–1216 (2004).
Takahashi, Y. et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J. Clin. Invest. 118, 1099–1109 (2008).
Carnevale-Schianca, F. et al. Allogeneic nonmyeloablative hematopoietic cell transplantation in metastatic colon cancer: tumor-specific T cells directed to a tumor-associated antigen are generated in vivo during GVHD. Blood 107, 3795–3803 (2006).
Orsini, E. et al. Expansion of tumor-specific CD8+ T cell clones in patients with relapsed myeloma after donor lymphocyte infusion. Cancer. Res. 63, 2561–2568 (2003).
Filatenkov, A. et al. Ineffective vaccination against solid tumors can be enhanced by hematopoietic cell transplantation. J. Immunol. 183, 7196–7203 (2009).
Gattinoni, L. et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202, 907–912 (2005).
Paulos, C. M. et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204 (2007).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011).
Wrzesinski, C. et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J. Immunother. 33, 1–7 (2010).
Mapara, M. Y. et al. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood 100, 1903–1909 (2002).
Mapara, M. Y., Kim, Y. M., Marx, J. & Sykes, M. Donor lymphocyte infusion-mediated graft-versus-leukemia effects in mixed chimeras established with a nonmyeloablative conditioning regimen: extinction of graft-versus-leukemia effects after conversion to full donor chimerism. Transplantation 76, 297–305 (2003).
Chakraverty, R. et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J. Exp. Med. 203, 2021–2031 (2006).
Sykes, M. et al. Mixed lymphohaemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone-marrow transplantation. Lancet 353, 1755–1759 (1999).
Mapara, M. Y. et al. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol. Blood Marrow Transplant. 12, 623–634 (2006).
Chakraverty, R. et al. The host environment regulates the function of CD8+ graft-versus-host-reactive effector cells. J. Immunol. 181, 6820–6828 (2008).
Flutter, B. et al. Nonhematopoietic antigen blocks memory programming of alloreactive CD8+ T cells and drives their eventual exhaustion in mouse models of bone marrow transplantation. J. Clin. Invest. 120, 3855–3868 (2010).
Kawai, T. et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 358, 353–361 (2008).
Scandling, J. D. et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am. J. Transplant. 12, 1133–1145 (2012).
Scandling, J. D. et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 358, 362–368 (2008).
Fudaba, Y. et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am. J. Transplant. 6, 2121–2133 (2006). These four clinical studies (references 106–109) collectively demonstrate that combined kidney and bone marrow transplantation can induce immune tolerance to HLA-mismatched and HLA-identical kidney allografts.
Tomita, Y., Khan, A. & Sykes, M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J. Immunol. 153, 1087–1098 (1994).
Kraus, A. B. et al. Early host CD8 T-cell recovery and sensitized anti-donor interleukin-2-producing and cytotoxic T-cell responses associated with marrow graft rejection following nonmyeloablative allogeneic bone marrow transplantation. Exp. Hematol. 31, 609–621 (2003).
Andreola, G. et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am. J. Transplant. 11, 1236–1247 (2011).
Liu, Y. P., Li, Z., Nador, R. G. & Strober, S. Simultaneous protection against allograft rejection and graft-versus-host disease after total lymphoid irradiation: role of natural killer T cells. Transplantation 85, 607–614 (2008).
Hongo, D., Tang, X., Dutt, S., Nador, R. G. & Strober, S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood 119, 1581–1589 (2012).
Leventhal, J. et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci. Transl. Med. 4, 124ra28 (2012).
Colson, Y. L., Shinde Patil, V. R. & Ildstad, S. T. Facilitating cells: novel promoters of stem cell alloengraftment and donor-specific transplantation tolerance in the absence of GVHD. Crit. Rev. Oncol. Hematol. 61, 26–43 (2007).
Yang, Y. G. & Sykes, M. Xenotransplantation: current status and a perspective on the future. Nature Rev. Immunol. 7, 519–531 (2007).
Ohdan, H., Yang, Y. G., Swenson, K. G., Kitamura, H. & Sykes, M. T cell and B cell tolerance to GALα1,3GAL-expressing heart xenografts is achieved in α1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Transplantation 71, 1532–1542 (2001).
Kawahara, T., Shimizu, I., Ohdan, H., Zhao, G. & Sykes, M. Differing mechanisms of early and late B cell hyporesponsiveness induced by mixed chimerism. Am. J. Transplant. 5, 2821–2829 (2005).
Kawahara, T., Rodriguez-Barbosa, J. I., Zhao, Y., Zhao, G. & Sykes, M. Global unresponsiveness as a mechanism of natural killer cell tolerance in mixed xenogeneic chimeras. Am. J. Transplant. 7, 2090–2097 (2007).
Griesemer, A. et al. Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation 17, 300–312 (2010).
Hugle, T. & Daikeler, T. Stem cell transplantation for autoimmune diseases. Haematologica 95, 185–188 (2010).
Milanetti, F., Abinun, M., Voltarelli, J. C. & Burt, R. K. Autologous hematopoietic stem cell transplantation for childhood autoimmune disease. Pediatr. Clin. North Am. 57, 239–271 (2010).
Daikeler, T. et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT autoimmune disease working party. Blood 118, 1693–1698 (2011).
de Kleer, I. et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood 107, 1696–1702 (2006).
Roord, S. T. A. et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood 111, 5233–5241 (2008).
Zhang, L., Bertucci, A. M., Ramsey-Goldman, R., Burt, R. K. & Datta, S. K. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-β-producing CD8+ Treg cells are associated with immunological remission of lupus. J. Immunol. 183, 6346–6358 (2009).
Muraro, P. A. et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 201, 805–816 (2005).
Daikeler, T. et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transplant. 44, 27–33 (2009).
Beilhack, G. F., Landa, R. R., Masek, M. A. & Shizuru, J. A. Prevention of type 1 diabetes with major histocompatibility complex-compatible and nonmarrow ablative hematopoietic stem cell transplants. Diabetes 54, 1770–1779 (2005).
Cho, S. G. et al. Immunoregulatory effects of allogeneic mixed chimerism induced by nonmyeloablative bone marrow transplantation on chronic inflammatory arthritis and autoimmunity in interleukin-1 receptor antagonist-deficient mice. Arthritis. Rheum. 54, 1878–1887 (2006).
Nikolic, B. et al. Mixed hematopoietic chimerism allows cure of autoimmune diabetes through allogeneic tolerance and reversal of autoimmunity. Diabetes 53, 376–383 (2004).
Racine, J. et al. Induction of mixed chimerism with MHC-mismatched but not matched bone marrow transplants results in thymic deletion of host-type autoreactive T-cells in NOD mice. Diabetes 60, 555–564 (2011).
Smith-Berdan, S., Gille, D., Weissman, I. L. & Christensen, J. L. Reversal of autoimmune disease in lupus-prone New Zealand black/New Zealand white mice by nonmyeloablative transplantation of purified allogeneic hematopoietic stem cells. Blood 110, 1370–1378 (2007).
Takeuchi, E., Shinohara, N. & Takeuchi, Y. Cognate interaction plays a key role in the surveillance of autoreactive B cells in induced mixed bone marrow chimerism in BXSB lupus mice. Autoimmunity 44, 363–372 (2011).
Bornhañuser, M., Aringer, M. & Thiede, C. Mixed lymphohematopoietic chimerism and response in Wegener's Granulomatosis. N. Engl. J. Med. 362, 2431–2432 (2010).
Burt, R. K. et al. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis. Rheum. 50, 2466–2470 (2004).
Jones, O. & Cahill, R. Nonmyeloablative allogeneic bone marrow transplantation of a child with systemic autoimmune disease and lung vasculitis. Immunol. Res. 41, 26–33 (2008).
Loh, Y. et al. Non-myeloablative allogeneic hematopoietic stem cell transplantation for severe systemic sclerosis: graft-versus-autoimmunity without graft-versus-host disease? Bone Marrow Transplant. 39, 435–437 (2007).
Sykes, M. & Nikolic, B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature 435, 620–627 (2005).
Lee, S. J. et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110, 4576–4583 (2007).
Petersdorf, E. W. & Hansen, J. A. New advances in hematopoietic cell transplantation. Curr. Opin. Hematol. 15, 549–554 (2008).
Kawase, T. et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood 110, 2235–2241 (2007).
Acknowledgements
We thank B. Sprangers for helpful review of the manuscript and S. Washington for expert assistance. This work was supported by grants from the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Cytokine-mobilized peripheral blood
-
Refers to treatment with granulocyte colony-stimulating factor and/or a stromal cell-derived factor 1 inhibitor to mobilize haematopoietic stem and progenitor cells from the marrow to the circulation, where they can be collected by leukapheresis for use in transplantation.
- Umbilical cord blood
-
(UCB). Blood that is collected from the umbilical cord after childbirth and is used as a source of haematopoietic stem cells to treat patients with haematological disorders. One unit of cord blood refers to blood harvested from one umbilical cord.
- Allogeneic
-
Of, or relating to, the same species. Allogeneic transplants are transplants between individuals of the same species. Mismatched MHC or minor histocompatibility antigens exist between the donor and the host, and this can elicit an immune response, which results in either graft rejection or graft-versus-host disease.
- Graft-versus-host disease
-
(GVHD). A pathological process mediated by host alloantigen-activated donor T cells attacking the normal tissues (mainly skin, gut and liver) of the host.
- Non-myeloablative conditioning
-
Also known as reduced-intensity conditioning. A less-intensive conditioning protocol that allows the engraftment of donor cells without ablating the host's haematopoietic system. Haematopoietic recovery occurs without engraftment of infused haematopoietic cells.
- Autologous
-
In autologous transplantation, the infused haematopoietic cells are from the patients themselves.
- Haploidentical
-
A haploidentical donor is matched for half of the MHC alleles of the recipient (one HLA haplotype). The donor can be the patient's parents, siblings, children or other relatives. Because as many as half of the alleles are mismatched, specific treatment of the patient and processing of the haematopoietic stem-cell graft are required to avoid severe immunological consequences
- Mesenchymal stem cells
-
(MSCs). Multipotent stem cells that can differentiate into various types of stromal cells.
- Myeloablative conditioning
-
Refers to intensive measures used to prepare hosts for allogeneic haematopoietic cell transplantation. It can include high doses of total body irradiation and/or chemotherapy to destroy pre-existing malignant cells and host immune cells. These highly intensive measures ablate normal haematopoietic cells of the host, which require the infusion of haematopoietic cells to prevent haematopoietic failure.
- Killer-cell immunoglobulin-like receptors
-
(KIRs). These receptors, encoded on human chromosome 19, are expressed by natural killer cell subsets and by a minor population of T cells. Inhibitory KIRs have locus and allele specificity for MHC class I molecules.
- Cyclophosphamide
-
A DNA-alkylating agent that is used widely as an antitumour agent or an immunosuppressive agent. Cyclophosphamide has been shown to destroy certain subsets of lymphocytes preferentially, including B cells and regulatory cells.
- Anti-thymocyte globulin
-
(ATG). Polyclonal antibodies against human T cells that are produced by immunizing rabbits or horses.
- Photodynamic purging
-
Refers to the process of deleting cells by introducing light-sensitive chemicals into the target cells, followed by exposure of these cells to light so that the chemicals cause the death of the target cells.
- Suicide gene
-
A gene whose product can induce cell death by apoptosis.
- Ganciclovir
-
An antiviral medication used to treat or prevent cytomegalovirus infections.
- Exhaustion
-
Impaired ability of effector T cells to carry out their functions, such as cytotoxicity and cytokine secretion, owing to chronic stimulation by antigens.
- Haemoglobinopathies
-
Diseases caused by genetic defects that lead to one of the globin chains of the haemoglobin molecule developing an abnormal structure.
- Xenotransplantation
-
Transplantation of tissue between foreign species.
- Natural antibody
-
Antibodies that are detected in the sera of normal individuals without any previous sensitization to the antigen. They can bind pathogens and self-antigens, such as ABO blood-group antigens.
Rights and permissions
About this article
Cite this article
Li, H., Sykes, M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 12, 403–416 (2012). https://doi.org/10.1038/nri3226
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3226
This article is cited by
-
Nuclear Tubulin Enhances CXCR4 Transcription and Promotes Chemotaxis Through TCF12 Transcription Factor in human Hematopoietic Stem Cells
Stem Cell Reviews and Reports (2023)
-
Extracorporeal photopheresis in acute and chronic steroid‑refractory graft-versus-host disease: an evolving treatment landscape
Leukemia (2022)
-
Association between employment status change and depression and anxiety in allogeneic stem cell transplant caregivers
Journal of Cancer Survivorship (2022)
-
Oral manifestations of graft-versus-host disease in patients submitted to allogeneic hematopoietic stem cell transplantation: the experience of a Brazilian Cancer Center
Supportive Care in Cancer (2022)
-
A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia
Nature Biomedical Engineering (2020)