Key Points
-
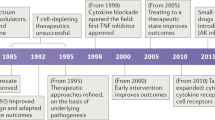
First-generation therapeutic antibodies have provided valuable lessons. A large number of therapeutic antibodies using diverse platforms have provided valuable insights into the strengths and limitations of each technology platform.
-
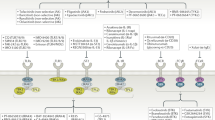
The mechanisms by which therapeutic antibodies mediate their functions are diverse.
-
Therapeutic antibodies operate through distinct mechanisms of action that affect their pharmacodynamic properties and biological activities.
-
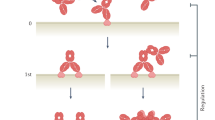
Effector function modifications can be used to optimize desired activities of a therapeutic antibody.
-
Increased and decreased binding affinities to Fc receptors for IgG (FcγRs), complement components and neonatal FcR can be used to optimize desired activities of therapeutic antibodies.
-
Bispecific and multispecific antibodies enhance therapeutic applications.
-
The development of multi-antigen-binding therapeutic antibodies enhances their therapeutic potential.
Abstract
The development of therapeutic antibodies has evolved over the past decade into a mainstay of therapeutic options for patients with autoimmune and inflammatory diseases. Substantial advances in understanding the biology of human diseases have been made and tremendous benefit to patients has been gained with the first generation of therapeutic antibodies. The lessons learnt from these antibodies have provided the foundation for the discovery and development of future therapeutic antibodies. Here we review how key insights obtained from the development of therapeutic antibodies complemented by newer antibody engineering technologies are delivering a second generation of therapeutic antibodies with promise for greater clinical efficacy and safety.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Reichert, J. M. Antibodies to watch in 2010. mAbs 2, 28–45 (2010). An excellent overview of therapeutic antibodies in Phase III clinical trials and beyond.
Scolnik, P. A. mAbs a business perspective. mAbs 1, 179–184 (2009).
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Almagro, J. C. & Fransson, J. Humanization of antibodies. Front. Biosci. 13, 1619–1633 (2008).
Reichert, J. M. Monoclonal antibodies as innovative therapeutics. Curr. Pharm. Biotechnol. 9, 423–430 (2008).
Lonberg, N. Fully human antibodies from transgenic mouse and phage display platforms. Curr. Opin. Immunol. 20, 450–459 (2008).
Mondon, P., Dubreuil, O., Bouayadi, K. & Kharrat, H. Human antibody libraries: a race to engineer and explore a larger diversity. Front. Biosci. 13, 1117–1129 (2008).
Parren, P. W. & van de Winkel, J. G. An integrated science-based approach to drug development. Curr. Opin. Immunol. 20, 426–430 (2008).
Kelley, B. Industralization of mAb production technology. The bioprocess industry at a crossroads. mAbs 1, 443–452 (2009).
Carter, P. J. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2006).
Kubota, T. et al. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 100, 1566–1572 (2009).
Presta, L. G. Molecular engineering and design of therapeutic antibodies. Curr. Opin. Immunol. 20, 460–470 (2008). References 11 and 12 provide a detailed summary of Fc engineering to enhance ADCC and CDC activity of IgG, as well as to extend serum half-life.
Targan, S. R. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. Crohn's Disease cA2 Study Group. N. Engl. J. Med. 337, 1029–1035 (1997).
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117, 244–279 (2008).
Baert, F. et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N. Engl. J. Med. 348, 601–608 (2003).
Hanauer, S. B. et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 359, 1541–1549 (2002).
Vultaggio, A. et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 27 Nov 2009 (doi: 10.1111/j.1398-9995.2009.02280.x).
Radstake, T. R. et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann. Rheum. Dis. 68, 1739–1745 (2009).
Weinblatt, M. E. et al. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 48, 35–45 (2003).
Bartelds, G. M. et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann. Rheum. Dis. 66, 921–926 (2007).
Emery, P. et al. Golimumab, a human anti-tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 60, 2272–2283 (2009).
Lugering, A. et al. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn's disease by using a caspase-dependent pathway. Gastroenterol. 121, 1145–1157 (2001).
Scallon, B. J., Moore, M. A., Trinh, H., Knight, D. M. & Ghrayeb, J. Chimeric anti-TNF-α momoclonal cA2 binds recombinant transmembrane TNF-α and activates immune effector functions. Cytokine 7, 251–259 (1995).
Van den Brande, J. M. et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterol. 124, 1774–1785 (2003).
Fossati, G. & Nesbitt, A. M. Effect of the anti-TNF agents, adalimumab, etanercept, infliximab, and certolizumab PEGOL (CDP870) on the induction of apoptosis in activated peripheral blood lymphocytes and monocytes. Am. J. Gastroenterol. 100, S298–S299 (2005).
Sandborn, W. J. et al. Certolizumab pegol for the treatment of Crohn's disease. N. Engl. J. Med. 357, 228–238 (2007).
Schreiber, S. et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N. Engl. J. Med. 357, 239–250 (2007).
Hawkins, P. N., Lachmann, H. J., Aganna, E. & McDermott, M. F. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 50, 607–612 (2004).
Lachmann, H. J. et al. In vivo regulation of interleukin 1β in patients with cryopyrin-associated periodic syndromes. J. Exp. Med. 206, 1029–1036 (2009).
Leonardi, C. L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
Papp, K. A. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371, 1675–1684 (2008).
Sandborn, W. J. et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterol. 135, 1130–1141 (2008).
Guttman-Yassky, E. et al. Blockade of CD11a by efalizumab in psoriasis patients induces a unique state of T-cell hyporesponsiveness. J. Invest. Dermatol. 128, 1182–1191 (2008).
Bousquet, J. et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 60, 302–308 (2005).
Beck, L. A., Marcotte, G. V., MacGlashan, D., Togias, A. & Saini, S. Omalizumab-induced reductions in mast cell Fcɛ RI expression and function. J. Allergy Clin. Immunol. 114, 527–530 (2004).
MacGlashan D. W. Jr et al. Down-regulation of FcɛRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J. Immunol. 158, 1438–1445 (1997).
Prussin, C. et al. Omalizumab treatment downregulates dendritic cell FcɛRI expression. J. Allergy Clin. Immunol. 112, 1147–1154 (2003).
MacGlashan, D. W. Jr. Endocytosis, recycling, and degradation of unoccupied FcɛRI in human basophils. J. Leukoc. Biol. 82, 1003–1010 (2007).
Blume, J. W., Kiratseavee, S. & Sands, M. F. Resolution of angioedema with omalizumab. J. Allergy Clin. Immunol. 119, S274 (2007).
Gober, L. M., Sterba, P. M., Eckman, J. A. & Saini, S. S. Effect of anti-IgE (omalizumab) in chronic idiopathic urticaria (CIU) patients. J. Allergy Clin. Immunol. 121, S147 (2008).
Shapiro, C. A., Kapatanos, N. S. & Sarmiento, E. Successful treatment of chronic idiopathic urticaria and angioedema (CIU) with Xolair (omalizumab). J. Allergy Clin. Immunol. 119, S274 (2007).
Eckman, J. A., Hamilton, R. G., Gober, L. M., Sterba, P. M. & Saini, S. S. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J. Investig. Dermatol. 128, 1956–1963 (2008).
Ferrer, M., Kinet, J. P. & Kaplan, A. P. Comparative studies of functional and binding assays for IgG anti-FcɛR1α (α-subunit) in chronic urticaria. J. Allergy Clin. Immunol. 101, 672–676 (1998).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
Coles, A. J. et al. Alemtuzumab vs. interferon β-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801 (2008).
Genovese, M. C. et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 58, 2652–2661 (2008).
Jacobi, A. M. et al. Differential effects of epratuzumab on peripheral blood B cells of patients with systemic lupus erythematosus versus normal controls. Ann. Rheum. Dis. 67, 450–457 (2008).
Golay, J. et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood 98, 3383–3389 (2001).
Gong, Q. et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J. Immunol. 174, 817–826 (2005).
Minard-Colin, V. et al. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcγRI, FcγRIII, and FcγRIV. Blood 112, 1205–1213 (2008).
Natsume, A., Shimizu-Yokoyama, Y., Satoh, M., Shitara, K. & Niwa, R. Engineered anti-CD20 antibodies with enhanced complement-activating capacity mediate potent anti-lymphoma activity. Cancer Sci. 100, 2411–2418 (2009).
Cartron, G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 (2002). This paper was the first to show an association between responsiveness to antibody therapy and a polymorphism in the gene encoding FcγRIII in patients, which strongly implicates an Fc or FcγR-mediated mechanism, such as ADCC, in the antitumour activity of rituximab.
Weng, W. K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Anolik, J. H. et al. The relationship of FcγRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 48, 455–459 (2003).
Johnson, N. A. et al. Diffuse large B-cell lymphoma: reduced CD20 expression is associated with an inferior survival. Blood 113, 3773–3780 (2009).
Olejniczak, S. H., Stewart, C. C., Donohue, K. & Czuczman, M. S. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol. Invest. 35, 93–114 (2006).
van Meerten, T., van Rijn, R. S., Hol, S., Hagenbeek, A. & Ebeling, S. B. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin. Cancer Res. 12, 4027–4035 (2006).
Di Gaetano, N. et al. Complement activation determines the therapeutic activity of rituximab in vivo. J. Immunol. 171, 1581–1587 (2003).
Weng, W. K. & Levy, R. Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. Blood 98, 1352–1357 (2001).
Kavanaugh, A. et al. Assessment of rituximab's immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann. Rheum. Dis. 67, 402–408 (2008).
Walsh, C. A., Fearon, U., FitzGerald, O., Veale, D. J. & Bresnihan, B. Decreased CD20 expression in rheumatoid arthritis synovium following 8 weeks of rituximab therapy. Clin. Exp. Rheumatol. 26, 656–658 (2008).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Smith, S. L. Ten years of orthoclone OKT3 (muromonab–CD3): a review. J. Transplant. Coord. 6, 109–121 (1996).
Norman, D. J., Chatenoud, L., Cohen, D., Goldman, M. & Shield, C. F. Consensus statement regarding OKT3-induced cytokine-release syndrome and human antimouse antibodies. Transplant. Proc. 25, 89–92 (1993).
Chatenoud, L. Anti-CD3 antibodies: towards clinical antigen-specific immunomodulation. Curr. Opin. Pharmacol. 4, 403–407 (2004).
Wiczling, P., Rosenzweig, M., Vaickus, L. & Jusko, W. J. Pharmacokinetics and pharmacodynamics of a chimeric/humanized anti-CD3 monoclonal antibody, otelixizumab (TRX4), in subjects with psoriasis and with type 1 diabetes mellitus. J. Clin. Pharmacol. 23 Nov 2009 (doi:10.1177/0091270009349376).
Herold, K. C. et al. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3γ1(Ala–Ala). J. Clin. Invest. 111, 409–418 (2003).
Bisikirska, B., Colgan, J., Luban, J., Bluestone, J. A. & Herold, K. C. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J. Clin. Invest. 115, 2904–2913 (2005).
Nelson, A. L. & Reichert, J. M. Development trends for therapeutic antibody fragments. Nature Biotechnol. 27, 331–337 (2009).
Labrijn, A. F., Aalberse, R. C. & Schuurman, J. When binding is enough: nonactivating antibody formats. Curr. Opin. Immunol. 20, 479–485 (2008). A comprehensive review of alternative strategies to generate antibodies that have minimized effector functions and that do not activate their targets.
Alegre, M. L. et al. A non-activating “humanized” anti-CD3 monoclonal antibody retains immunosuppressive properties in vivo. Transplantation 57, 1537–1543 (1994).
Woodle, E. S. et al. Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3γ1(Ala–Ala) in the treatment of acute renal allograft rejection. Transplantation 68, 608–616 (1999).
Bolt, S. et al. The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur. J. Immunol. 23, 403–411 (1993).
Salfeld, J. G. Isotype selection in antibody engineering. Nature Biotechnol. 25, 1369–1372 (2007).
van der Neut Kolfschoten, M. et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 (2007). This article shows that IgG4 can exchange Fab arms in vivo to form bispecific antibodies, and it identifies mutations for preventing this exchange.
Jefferis, R. Antibody therapeutics: isotype and glycoform selection. Expert Opin. Biol. Ther. 7, 1401–1413 (2007).
Bruhns, P. et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113, 3716–3725 (2009).
Reddy, M. P. et al. Elimination of Fc receptor-dependent effector functions of a modified IgG4 monoclonal antibody to human CD4. J. Immunol. 164, 1925–1933 (2000).
Rother, R. P., Rollins, S. A., Mojcik, C. F., Brodsky, R. A. & Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nature Biotechnol. 25, 1256–1264 (2007).
Davis, P. M., Abraham, R., Xu, L., Nadler, S. G. & Suchard, S. J. Abatacept binds to the Fc receptor CD64 but does not mediate complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. J. Rheumatol. 34, 2204–2210 (2007).
Kabat, E. A., Wu, T. T., Perry, H. M., Gottesman, K. S. & Foeller, C. Sequences of Proteins of Immunological Interest 5th edn (US National Institutes of Health, Bethesda, 1991).
Choy, E. H. et al. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatol. (Oxford) 41, 1133–1137 (2002).
Teeling, J. L. et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104, 1793–1800 (2004).
Shields, R. L. et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J. Biol. Chem. 276, 6591–6604 (2001). A detailed mutational mapping of human IgG1 Fc for FcγRs and FcRn. Fc variants described include ones that improve binding to FcγRIIIA and enhance ADCC.
Stavenhagen, J. B. et al. Enhancing the potency of therapeutic monoclonal antibodies via Fc optimization. Adv. Enzyme Regul. 48, 152–164 (2008).
Lazar, G. A. et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl Acad. Sci. USA 103, 4005–4010 (2006). The paper shows a broadly applicable Fc protein engineering strategy, used to create variants of several approved therapeutic antibodies with ∼100-fold more potent ADCC properties.
Richards, J. O. et al. Optimization of antibody binding to FcγRIIa enhances macrophage phagocytosis of tumor cells. Mol. Cancer Ther. 7, 2517–2527 (2008).
Jung, S. T. et al. Aglycosylated IgG variants expressed in bacteria that selectively bind FcγRI potentiate tumor cell killing by monocyte-dendritic cells. Proc. Natl Acad. Sci. USA 107, 604–609 (2010).
Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nature Rev. Drug Discov. 8, 226–234 (2009).
Umaña, P., Jean-Mairet, J., Moudry, R., Amstutz, H. & Bailey, J. E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nature Biotechnol. 17, 176–180 (1999). This is the first example of the alteration of the glycosylation of an Fc to enhance ADCC activity of IgG.
Shields, R. L. et al. Lack of fucose on human IgG1 N.-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003). A detailed analysis of the influence of Fc glycans on the ability of a humanized antibody to support ADCC, showing that the absence of fucose has the most important role in enhancing ADCC.
Yamane-Ohnuki, N. et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 87, 614–622 (2004).
Iida, S. et al. Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcγRIIIa. Clin. Cancer Res. 12, 2879–2887 (2006). This paper shows a broadly applicable Fc glyco-engineering strategy used to create variants of rituximab and trastuzumab with ∼100-fold more potent ADCC activity. Non-fucosylated IgG1, unlike fucosylated counterparts, largely evade the inhibitory effect of plasma IgG on ADCC.
Li, H. et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nature Biotechnol. 24, 210–215 (2006).
Idusogie, E. E. et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J. Immunol. 164, 4178–4184 (2000).
Idusogie, E. E. et al. Engineered antibodies with increased activity to recruit complement. J. Immunol. 166, 2571–2575 (2001).
Dall'Acqua, W. F., Cook, K. E., Damschroder, M. M., Woods, R. M. & Wu, H. Modulation of the effector functions of a human IgG1 through engineering of its hinge region. J. Immunol. 177, 1129–1138 (2006).
Natsume, A. et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 68, 3863–3872 (2008). This article describes a potent enhancement of CDC activity of IgG using a IgG1–IgG3 hybrid isotype.
Vugmeyster, Y. et al. Depletion of B cells by a humanized anti-CD20 antibody PRO70769 in Macaca fascicularis. J. Immunother. 28, 212–219 (2005).
Genovese, M. C. et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 58, 2652–2661 (2008).
Mössner, E. et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct- and immune effector cell-mediated B-cell cytotoxicity. Blood 1 Mar 2010 (doi:10.1182/blood-2009-06-225979).
Reed, J. L. et al. MEDI-563, a humanized anti-IL-5Ra antibody with enhanced effector function, induces reversible blood eosinopenia in mild asthmatics. J. Allergy Clin. Immunol. Abstr. 121, S47. (2009).
Beers, S. A. et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood 112, 4170–4177 (2008).
Ivanov, A. et al. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J. Clin. Invest. 119, 2143–2159 (2009). This manuscript describes a newly discovered mechanism for type II CD20-specific antibodies.
Lobo, E. D., Hansen, R. J. & Balthasar, J. P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 93, 2645–2668 (2004).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nature Rev. Immunol. 7, 715–725 (2007).
Morell, A., Terry, W. D. & Waldmann, T. A. Metabolic properties of IgG subclasses in man. J. Clin. Invest. 49, 673–680 (1970).
Ghetie, V. et al. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nature Biotechnol. 15, 637–640 (1997). This is the first demonstration of extended serum half-life of an IgG owing to engineering the Fc region for higher affinity binding to FcRn.
Hinton, P. R. et al. Engineered human IgG antibodies with longer serum half-lives in primates. J. Biol. Chem. 279, 6213–6216 (2004). This is the first demonstration of extended serum half-life of an IgG in non-human primates owing to Fc engineering to strengthen binding to FcRn, while maintaining pH-dependence of interaction.
Hinton, P. R. et al. An engineered human IgG1 antibody with longer serum half-life. J. Immunol. 176, 346–356 (2006).
Datta-Mannan, A., Witcher, D. R., Tang, Y., Watkins, J. & Wroblewski, V. J. Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J. Biol. Chem. 282, 1709–1717 (2007).
Dall'Acqua, W. F., Kiener, P. A. & Wu, H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J. Biol. Chem. 281, 23514–23524 (2006).
Oganesyan, V. et al. Structural characterization of a human Fc fragment engineered for extended serum half-life. Mol. Immunol. 46, 1750–1755 (2009).
Zalevsky, J. et al. Enhanced antibody half-life improves in vivo activity. Nature Biotechnol. 28, 157–159 (2010). This paper was the first to show that antibodies engineered for extended half-life can have enhanced efficacy in preclinical animal models.
Vaccaro, C., Bawdon, R., Wanjie, S., Ober, R. J. & Ward, E. S. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc. Natl Acad. Sci. USA 103, 18709–18714 (2006).
Petkova, S. B. et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int. Immunol. 18, 1759–1769 (2006).
Dall'Acqua, W. F. et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J. Immunol. 169, 5171–5180 (2002).
Vaccaro, C., Zhou, J., Ober, R. J. & Ward, E. S. Engineering the Fc region of immunoglobulin G. to modulate in vivo antibody levels. Nature Biotechnol. 23, 1283–1288 (2005).
Kenanova, V. et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv–Fc antibody fragments. Cancer Res. 65, 622–631 (2005).
Chapman, A. P. et al. Therapeutic antibody fragments with prolonged in vivo half-lives. Nature Biotechnol. 17, 780–783 (1999).
Veronese, F. M. & Mero, A. The impact of PEGylation on biological therapies. BioDrugs 22, 315–329 (2008).
Yeh, P. et al. Design of yeast-secreted albumin derivatives for human therapy: biological and antiviral properties of a serum albumin-CD4 genetic conjugate. Proc. Natl Acad. Sci. USA 89, 1904–1908 (1992).
Capon, D. J. et al. Designing CD4 immunoadhesins for AIDS therapy. Nature 337, 525–531 (1989).
Schellenberger, V. et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nature Biotechnol. 27, 1186–1190 (2009).
Dennis, M. S. et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J. Biol. Chem. 277, 35035–35043 (2002).
Holliger, P., Wing, M., Pound, J. D., Bohlen, H. & Winter, G. Retargeting serum immunoglobulin with bispecific diabodies. Nature Biotechnol. 15, 632–636 (1997).
Elliott, S. et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nature Biotechnol. 21, 414–421 (2003).
Carter, P. J. & Senter, P. D. Antibody-drug conjugates for cancer therapy. Cancer J. 14, 154–169 (2008).
Baeuerle, P. A. & Reinhardt, C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 69, 4941–4944 (2009).
Chames, P. & Baty, D. Bispecific antibodies for cancer therapy. Curr. Opin. Drug Discov. Devel. 12, 276–283 (2009).
Marvin, J. S. & Zhu, Z. Recombinant approaches to IgG-like bispecific antibodies. Acta Pharmacol. Sin. 26, 649–658 (2005).
Nisonoff, A. & Mandy, W. J. Quantitative estimation of the hybridization of rabbit antibodies. Nature 194, 355–359 (1962).
Kontermann, R. E. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol. Sin. 26, 1–9 (2005).
Staerz, U. D., Kanagawa, O. & Bevan, M. J. Hybrid antibodies can target sites for attack by T cells. Nature 314, 628–631 (1985).
Bargou, R. et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008).
Lu, D. et al. Complete inhibition of vascular endothelial growth factor (VEGF) activities with a bifunctional diabody directed against both VEGF kinase receptors, fms-like tyrosine kinase receptor and kinase insert domain-containing receptor. Cancer Res. 61, 7002–7008 (2001).
Lu, D. et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J. Biol. Chem. 280, 19665–19672 (2005).
Lu, D. et al. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J. Biol. Chem. 279, 2856–2865 (2004).
Bostrom, J. et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science 323, 1610–1614 (2009). This report describes how a 'two-in-one' antibody generated by phage display can bind either HER2 or VEGF with high affinity and inhibit tumour progression in mouse models. This is potentially broadly applicable to other antibodies and antigens.
Genovese, T. et al. Treatment with a novel poly(ADP-ribose) glycohydrolase inhibitor reduces development of septic shock-like syndrome induced by zymosan in mice. Crit. Care Med. 32, 1365–1374 (2004).
Weinblatt, M. et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann. Rheum. Dis. 66, 228–234 (2007).
Arend, W. P., Palmer, G. & Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 (2008).
van den Berg, W. B., Joosten, L. A., Helsen, M. & van de Loo, F. A. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin. Exp. Immunol. 95, 237–243 (1994).
Joosten, L. A., Helsen, M. M., van de Loo, F. A. & van den Berg, W. B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNFα, anti-IL-1α/β, and IL-1Ra. Arthritis Rheum. 39, 797–809 (1996).
Wu, C. et al. Molecular construction and optimization of anti-human IL-1α/β dual variable domain immunoglobulin (DVD-IgTM) molecules. mAbs 1, 339–347 (2009).
Wu, C. et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nature Biotechnol. 25, 1290–1297 (2007). This article was the first to describe a potentially broadly applicable bispecific antibody format (DVD-IgG), which was shown to simultaneously neutralize pairs of pro-inflammatory cytokines. A DVD-IgG binding IL-1α and IL-1β has similar in vivo potency to a combination of the two monospecific IgG molecules from which it was derived.
Louten, J., Boniface, K. & de Waal Malefyt, R. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 123, 1004–1011 (2009).
Mabry, R. et al. Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng. Des. Sel. 23, 115–127 (2009). This report identifies bispecific antibodies that potently neutralize both IL-17A and IL-23 with good stability and pharmacokinetic properties. These bispecific antibodies have potential use for the treatment of allergic diseases mediated by T H 17 cells.
Connelly, R. J. et al. Mitogenic properties of a bispecific single-chain Fv–Ig fusion generated from CD2-specific mAb to distinct epitopes. Int. Immunol. 10, 1863–1872 (1998).
Jendreyko, N. et al. Intradiabodies, bispecific, tetravalent antibodies for the simultaneous functional knockout of two cell surface receptors. J. Biol. Chem. 278, 47812–47819 (2003).
Coloma, M. J. & Morrison, S. L. Design and production of novel tetravalent bispecific antibodies. Nature Biotechnol. 15, 159–163 (1997).
Holliger, P. & Winter, G. Engineering bispecific antibodies. Curr. Opin. Biotechnol. 4, 446–449 (1993).
Petereit, H. F. & Rubbert-Roth, A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult. Scler. 15, 189–192 (2009).
Bird, R. E. et al. Single-chain antigen-binding proteins. Science 242, 423–426 (1988).
Huston, J. S. et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl Acad. Sci. USA 85, 5879–5883 (1988).
Holliger, P., Prospero, T. & Winter, G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc. Natl Acad. Sci. USA 90, 6444–6448 (1993).
Saerens, D., Ghassabeh, G. H. & Muyldermans, S. Single-domain antibodies as building blocks for novel therapeutics. Curr. Opin. Pharmacol. 8, 600–608 (2008).
Milstein, C. & Cuello, A. C. Hybrid hybridomas and their use in immunohistochemistry. Nature 305, 537–540 (1983).
Suresh, M. R., Cuello, A. C. & Milstein, C. Bispecific monoclonal antibodies from hybrid hybridomas. Methods Enzymol. 121, 210–228 (1986).
Merchant, A. M. et al. An efficient route to human bispecific IgG. Nature Biotechnol. 16, 677–681 (1998). This paper describes the first efficient and broadly applicable method for preparing bispecific human IgG potentially suitable for human therapy.
Isaacs, J. D. Antibody engineering to develop new antirheumatic therapies. Arthritis Res. Ther. 11, 225 (2009).
Oganesyan, V., Gao, C., Shirinian, L., Wu, H & Dall'Acqua, W. F. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr. D Biol. Crystallogr. 64, 700–704 (2008).
Weiner, L. M., Surana, R. & Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Rev. Immunol. 10, 317–327 (2010).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
A.C.C. and P.J.C. are employees of Genentech, Inc.
Related links
Glossary
- Effector functions
-
Fc-mediated antibody properties that are involved in target cell destruction: antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC).
- Half-life
-
The time taken for the plasma concentration of a drug to fall to half of its original value. Initial half-life and terminal half-life refer to the first (distribution) and second (elimination) phase for bi-exponential pharmacokinetics, respectively.
- Phage display
-
Technology for displaying a protein, such as an antibody fragment, on the surface of a bacteriophage that contains the gene (or genes) encoding the displayed protein (or proteins), thereby physically linking the genotype and phenotype.
- Binding affinity
-
For two interacting molecules this is the ratio of their association (ka) and dissociation (kd) rate constants: Kd = kd ÷ ka.
- Neonatal FcR
-
(FcRn). An Fc receptor that is structually related to MHC class I molecules and protects IgG from degradation, resulting in long serum half-life. Additionally, FcRn mediates IgG transfer from a mother to her fetus, thereby providing passive immunity.
- Immunoadhesin
-
A fusion protein that combines the functional domain of a binding protein, such as a receptor or ligand, with an immunoglobulin Fc domain. Such Fc fusion proteins can endow binding proteins with antibody-like properties including long serum half-life and effector functions.
- Human anti-chimeric antibodies
-
(HACAs). The immune system can develop an antibody response to chimeric antibodies. Binding of HACAs to chimeric antibodies forms immune complexes that can shorten the half-life of the therapeutic antibodies and compromise clinical effectiveness. Additionally these immune complexes can deposit in organs such as skin and kidney causing adverse events, including rashes and glomerulonephritis.
- Human anti-human antibodies
-
(HAHAs). The immune system can also develop an immune response to human therapeutic antibodies. HAHAs tend to be less common and less severe than HACAs.
- Bispecific antibodies
-
Antibodies capable of binding to two different antigens or two distinct epitiopes on the same antigen are known as bispecific.
- Exposure
-
In the pharmacokinetic sense, the area under the curve for a plot of drug concentration versus time.
- Valency
-
For antibodies, including bispecific antibodies, the number of binding sites for each cognate antigen.
Rights and permissions
About this article
Cite this article
Chan, A., Carter, P. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 10, 301–316 (2010). https://doi.org/10.1038/nri2761
Issue Date:
DOI: https://doi.org/10.1038/nri2761
This article is cited by
-
GluN2B subunit selective N-methyl-D-aspartate receptor ligands: Democratizing recent progress to assist the development of novel neurotherapeutics
Molecular Diversity (2023)
-
Development and Characterization of a Novel Single-Chain Antibody Against B-Cell Activating Factor
Molecular Biotechnology (2023)
-
A New Approach to the Management of COVID-19. Antagonists of IL-6: Siltuximab
Advances in Therapy (2022)
-
COVID-19 one year into the pandemic: from genetics and genomics to therapy, vaccination, and policy
Human Genomics (2021)
-
Ab locks for improving the selectivity and safety of antibody drugs
Journal of Biomedical Science (2020)