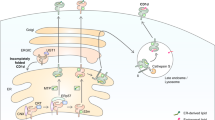
Key Points
-
Lipid antigens are immunogenic for T cells when they are presented by CD1 antigen-presenting molecules that bind lipids. Five CD1 proteins are expressed in humans, whereas only one is expressed in mice.
-
Lipid molecules are not soluble in water and are always associated with membranes or lipid-binding proteins in tissues and biological fluids. This characteristic makes the biology and immunogenicity of lipids different from that of peptides. Microbial and self lipid antigens have been identified.
-
Intracellular microorganisms that infect antigen-presenting cells and localize in the phagosomes release immunogenic lipids, which travel to the compartments where they intersect with recycling CD1 molecules.
-
Complex glycolipids become stimulatory after processing and loading onto CD1 molecules in endosomal compartments. Both of these events are assisted by lipid-transfer proteins, which are necessary and redundant.
-
Lipid-specific T cells can use both T-cell receptor (TCR)-αβ and TCR-γδ, do not preferentially express CD4 or CD8 co-receptors, are selected intrathymically, and are primed in the periphery. Recall lipid-specific T-cell responses are observed after microbial infections.
-
Lipid-specific T cells release either T helper 1 (TH1)- or TH2-type cytokines, and might have regulatory functions. They therefore participate in immune responses with the same qualification as peptide-specific T cells.
-
The response to lipid antigens is associated with previous or ongoing bacterial infections, the recognition of tumour cells and the control of autoimmunity.
Abstract
Recent studies have shown that the recognition of lipid antigens by the immune system is important for defence against infection and other diseases, and that lipid-specific responses occur at higher frequencies than previously suspected. Thanks to several recent advances in this field, we now have a better appreciation of the molecular and cellular requirements of T-cell stimulation by lipids. These findings have raised new questions about the mechanisms of lipid presentation, the priming and clonal expansion of lipid-specific T cells, and their differentiation into memory cells. A greater understanding of lipid-specific T cells and the molecular mechanisms of lipid immunogenicity should facilitate the development of lipid-based vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Porcelli, S. A. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59, 1–98 (1995).
Calabi, F., Jarvis, J. M., Martin, L. & Milstein, C. Two classes of CD1 genes. Eur. J. Immunol. 19, 285–292 (1989).
Zeng, Z. et al. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 277, 339–345 (1997).
Gadola, S. D. et al. Structure of human CD1b with bound ligands at 2.3Å, a maze for alkyl chains. Nature Immunol. 3, 721–726 (2002).
Zajonc, D. M., Elsliger, M. A., Teyton, L. & Wilson, I. A. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15Å. Nature Immunol. 4, 808–815 (2003).
Zajonc, D. M. et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity 22, 209–219 (2005). References 3–6 present crystal structure analyses of mouse CD1d and human CD1b and CD1a, which illustrate the molecular bases for lipid–CD1 interactions.
Moody, D. B., Zajonc, D. M. & Wilson, I. A. Anatomy of CD1–lipid–antigen complexes. Nature Rev. Immunol. 5, 1–14 (2005).
Shamshiev, A. et al. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 195, 1013–1021 (2002). This paper shows that sulphatide is a promiscuous self glycolipid that binds to CD1a, CD1b and CD1c, and that the immunogenic complexes that are generated by sulphatide have differing half-lives in vivo depending on the CD1 molecule.
Bendelac, A., Rivera, M. N., Park, S. H. & Roark, J. H. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15, 535–562 (1997).
Kronenberg, M. & Gapin, L. The unconventional lifestyle of NKT cells. Nature Rev. Immunol. 2, 557–568 (2002).
Taniguchi, M., Harada, M., Kojo, S., Nakayama, T. & Wakao, H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21, 483–513 (2003).
Godfrey, D. I. & Kronenberg, M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
Van Kaer, L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nature Rev. Immunol. 5, 31–42 (2005).
Beckman, E. M. et al. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372, 691–694 (1994). This was the first study to show that lipids stimulate T cells.
Grant, E. P. et al. Fine specificity of TCR complementarity-determining region residues and lipid antigen hydrophilic moieties in the recognition of a CD1–lipid complex. J. Immunol. 168, 3933–3940 (2002).
Sieling, P. A. et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227–230 (1995).
Moody, D. B. et al. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J. Exp. Med. 192, 965–976 (2000). This paper reports that pathogenic mycobacteria might use sugars from the infected host to generate pathogen-specific glycolipid antigens. This mechanism might focus the immune response against cells that are infected with virulent bacilli.
Sada, E., Brennan, P. J., Herrera, T. & Torres, M. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28, 2587–2590 (1990).
van Kooyk, Y. & Geijtenbeek, T. B. DC-SIGN: escape mechanism for pathogens. Nature Rev. Immunol. 3, 697–709 (2003).
Gilleron, M. et al. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 199, 649–659 (2004). This study identifies a new and highly immunogenic mycobacterial glycolipid, which is expressed mainly by virulent bacilli and induces a strong response only in previously infected hosts.
Moody, D. B. et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404, 884–888 (2000).
Matsunaga, I. et al. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J. Exp. Med. 200, 1559–1569 (2004).
Fairhurst, R. M., Wang, C. X., Sieling, P. A., Modlin, R. L. & Braun, J. CD1-restricted T cells and resistance to polysaccharide-encapsulated bacteria. Immunol. Today 19, 257–259 (1998).
Mattner, J. et al. Both exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Kawahara, K., Kuraishi, H. & Zahringer, U. Chemical structure and function of glycosphingolipids of Sphingomonas spp. and their distribution among members of the α-4 subclass of Proteobacteria. J. Ind. Microbiol. Biotechnol. 23, 408–413 (1999).
Brigl, M., Bry, L., Kent, S. C., Gumperz, J. E. & Brenner, M. B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nature Immunol. 4, 1230–1237 (2003).
Amprey, J. L. et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200, 895–904 (2004).
Pomorski, T., Hrafnsdottir, S., Devaux, P. F. & van Meer, G. Lipid distribution and transport across cellular membranes. Semin. Cell. Dev. Biol. 12, 139–148 (2001).
Hsu, F. F., Bohrer, A. & Turk, J. Electrospray ionization tandem mass spectrometric analysis of sulfatide. Determination of fragmentation patterns and characterization of molecular species expressed in brain and in pancreatic islets. Biochim. Biophys. Acta 1392, 202–216 (1998).
Hakomori, S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem. 50, 733–764 (1981).
Jahng, A. et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199, 947–957 (2004).
Shamshiev, A. et al. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29, 1667–1675 (1999). This was the first observation that self glycolipids can stimulate T cells. Patients with multiple sclerosis showed increased numbers of circulating self-reactive T cells that were specific for different glycosphingolipids. The implications for autoimmune diseases are discussed in this paper.
Shamshiev, A. et al. The αβ T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity 13, 255–264 (2000). This paper describes how the loading of lipid antigens can occur on the cell surface and how modifications in the lipid structures of glycosphingolipids affect the T-cell response.
Wu, D. Y., Segal, N. H., Sidobre, S., Kronenberg, M. & Chapman, P. B. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198, 173–181 (2003).
Gumperz, J. E. et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12, 211–221 (2000).
Moody, D. B. et al. T cell activation by lipopeptide antigens. Science 303, 527–531 (2004). Using the example of the lipopeptide didehydroxymycobactin, this important paper shows that lipoproteins can also be specifically recognized by CD1-restricted T cells.
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998).
Van Rhijn, I. et al. CD1d-restricted T cell activation by nonlipidic small molecules. Proc. Natl Acad. Sci. USA 101, 13578–13583 (2004).
Moody, D. B. et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 278, 283–286 (1997).
LaLonde, J. M., Bernlohr, D. A. & Banaszak, L. J. The up-and-down β-barrel proteins. FASEB J. 8, 1240–1247 (1994).
Rauch, J. et al. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J. Biol. Chem. 278, 47508–47515 (2003).
Melian, A. et al. Molecular recognition of human CD1b antigen complexes: evidence for a common pattern of interaction with αβ TCRs. J. Immunol. 165, 4494–4504 (2000).
Ernst, W. A. et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity 8, 331–340 (1998).
Moody, D. B. et al. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nature Immunol. 3, 435–442 (2002).
Tailleux, L. et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127 (2003).
Schaible, U. E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nature Med. 9, 1039–1046 (2003).
Prigozy, T. I. et al. Glycolipid antigen processing for presentation by CD1d molecules. Science 291, 664–667 (2001). This was the first and only study to show that glycolipids might require processing to become immunogenic.
Sugita, M., Porcelli, S. A. & Brenner, M. B. Assembly and retention of CD1b heavy chains in the endoplasmic reticulum. J. Immunol. 159, 2358–2365 (1997).
Huttinger, R., Staffler, G., Majdic, O. & Stockinger, H. Analysis of the early biogenesis of CD1b: involvement of the chaperones calnexin and calreticulin, the proteasome and β2-microglobulin. Int. Immunol. 11, 1615–1623 (1999).
Kang, S. J. & Cresswell, P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J. Biol. Chem. 277, 44838–44844 (2002).
De Silva, A. D. et al. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J. Immunol. 168, 723–733 (2002).
Jayawardena-Wolf, J. & Bendelac, A. CD1 and lipid antigens: intracellular pathways for antigen presentation. Curr. Opin. Immunol. 13, 109–113 (2001).
Briken, V., Jackman, R. M., Dasgupta, S., Hoening, S. & Porcelli, S. A. Intracellular trafficking pathway of newly synthesized CD1b molecules. EMBO J. 21, 825–834 (2002).
Jayawardena-Wolf, J., Benlagha, K., Chiu, Y. H., Mehr, R. & Bendelac, A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity 15, 897–908 (2001).
Kang, S. J. & Cresswell, P. Regulation of intracellular trafficking of human CD1d by association with MHC class II molecules. EMBO J. 21, 1650–1660 (2002).
Sugita, M., Peters, P. J. & Brenner, M. B. Pathways for lipid antigen presentation by CD1 molecules: nowhere for intracellular pathogens to hide. Traffic 1, 295–300 (2000).
Sprong, H., van der Sluijs, P. & van Meer, G. How proteins move lipids and lipids move proteins. Nature Rev. Mol. Cell Biol. 2, 504–513 (2001). This outstanding review simplifies the diverse and complex aspects of lipid transport and interactions with proteins.
Beatty, W. L. et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1, 235–247 (2000).
Schaible, U. E., Hagens, K., Fischer, K., Collins, H. L. & Kaufmann, S. H. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J. Immunol. 164, 4843–4852 (2000).
Zhou, D. et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science 303, 523–527 (2004).
Kang, S. J. & Cresswell, P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nature Immunol. 5, 175–181 (2004).
Winau, F. et al. Saposin C is required for lipid presentation by human CD1b. Nature Immunol. 5, 169–174 (2004). References 61–63 describe the role of saposins and the GM2-activator in facilitating presentation to CD1-restricted T cells, possibly by promoting lipid mobilization, digestion by hydroxylases and loading on CD1 molecules. This complex series of events is referred to as lipid editing.
Sandhoff, K. & Kolter, T. Biosynthesis and degradation of mammalian glycosphingolipids. Philos. Trans. R. Soc. Lond. B 358, 847–861 (2003).
De Libero, G. Immunology. The Robin Hood of antigen presentation. Science 303, 485–487 (2004).
Brozovic, S. et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nature Med. 10, 535–539 (2004).
Joyce, S. et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 279, 1541–1544 (1998).
MacDonald, H. R. Development and selection of NKT cells. Curr. Opin. Immunol. 14, 250–254 (2002).
Brigl, M. & Brenner, M. B. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 (2004).
Berzins, S. P. et al. Parallels and distinctions between T and NKT cell development in the thymus. Immunol. Cell Biol. 82, 269–275 (2004).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 26, 877–900 (2005).
Coles, M. C. & Raulet, D. H. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164, 2412–2418 (2000).
Forestier, C. et al. T cell development in mice expressing CD1d directed by a classical MHC class II promoter. J. Immunol. 171, 4096–4104 (2003).
Elewaut, D. et al. NIK-dependent RelB activation defines a unique signaling pathway for the development of Vα14i NKT cells. J. Exp. Med. 197, 1623–1633 (2003).
Schmidt-Supprian, M. et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-κB activation. Proc. Natl Acad. Sci. USA 101, 4566–4571 (2004).
Sivakumar, V., Hammond, K. J., Howells, N., Pfeffer, K. & Weih, F. Differential requirement for Rel/nuclear factorκB family members in natural killer T cell development. J. Exp. Med. 197, 1613–1621 (2003).
Stanic, A. K. et al. NF-κB controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J. Immunol. 172, 2265–2273 (2004).
Townsend, M. J. et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20, 477–494 (2004).
Borowski, C. & Bendelac, A. Signaling for NKT cell development: the SAP–FynT connection. J. Exp. Med. 201, 833–836 (2005).
Nichols, K. E. et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nature Med. 11, 340–345 (2005).
Chung, B., Aoukaty, A., Dutz, J., Terhorst, C. & Tan, R. Cutting edge: signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J. Immunol. 174, 3153–3157 (2005).
Pasquier, B. et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 201, 695–701 (2005).
Bendelac, A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995).
Elewaut, D. et al. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Vα14i NKT cells. J. Exp. Med. 198, 1133–1146 (2003).
Cernadas, M. et al. Lysosomal localization of murine CD1d mediated by AP-3 is necessary for NK T cell development. J. Immunol. 171, 4149–4155 (2003).
Honey, K. et al. Thymocyte expression of cathepsin L is essential for NKT cell development. Nature Immunol. 3, 1069–1074 (2002).
Sandhoff, K., Kolter, T. & Harzar, K. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C., Beaudet, A. L., Sly, W. S. & Valle, D.) 3371–3388 (McGraw-Hill, New York, 2001).
Voyle, R. B. et al. Ligand-dependent inhibition of CD1d-restricted NKT cell development in mice transgenic for the activating receptor Ly49D. J. Exp. Med. 197, 919–925 (2003).
Hayakawa, Y., Berzins, S. P., Crowe, N. Y., Godfrey, D. I. & Smyth, M. J. Antigen-induced tolerance by intrathymic modulation of self-recognizing inhibitory receptors. Nature Immunol. 5, 590–596 (2004).
Ulrichs, T., Moody, D. B., Grant, E., Kaufmann, S. H. & Porcelli, S. A. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infect. Immun. 71, 3076–3087 (2003).
Hiromatsu, K. et al. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. J. Immunol. 169, 330–339 (2002).
Dascher, C. C. et al. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int. Immunol. 15, 915–925 (2003).
Kawashima, T. et al. Cutting edge: major CD8 T cell response to live bacillus Calmette-Guerin is mediated by CD1 molecules. J. Immunol. 170, 5345–5348 (2003).
Sieling, P. A. et al. Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J. Immunol. 164, 4790–4796 (2000).
Bendelac, A., Hunziker, R. D. & Lantz, O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 184, 1285–1293 (1996).
Miyamoto, K., Miyake, S. & Yamamura, T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534 (2001).
Gumperz, J. E., Miyake, S., Yamamura, T. & Brenner, M. B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195, 625–636 (2002).
Akbari, O. et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Med. 9, 582–588 (2003).
Fuss, I. J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical TH2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Grant, E. P. et al. Molecular recognition of lipid antigens by T cell receptors. J. Exp. Med. 189, 195–205 (1999).
Porcelli, S. et al. Recognition of cluster of differentiation 1 antigens by human CD4–CD8–cytolytic T lymphocytes. Nature 341, 447–450 (1989). This was the first report on CD1-restricted TCR-αβ and TCR-γδ T cells.
Faure, F., Jitsukawa, S., Miossec, C. & Hercend, T. CD1c as a target recognition structure for human T lymphocytes: analysis with peripheral blood γ/δ cells. Eur. J. Immunol. 20, 703–706 (1990).
Spada, F. M. et al. Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J. Exp. Med. 191, 937–948 (2000).
Exley, M., Porcelli, S., Furman, M., Garcia, J. & Balk, S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant Vα24JαQT cell receptor α chains. J. Exp. Med. 188, 867–876 (1998).
Das, H. et al. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity 15, 83–93 (2001).
Stenger, S. & Modlin, R. L. T cell mediated immunity to Mycobacterium tuberculosis. Curr. Opin. Microbiol. 2, 89–93 (1999).
Smyth, M. J. et al. NKT cells — conductors of tumor immunity? Curr. Opin. Immunol. 14, 165–171 (2002).
Metelitsa, L. S., Weinberg, K. I., Emanuel, P. D. & Seeger, R. C. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia 17, 1068–1077 (2003).
Dhodapkar, M. V. et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 197, 1667–1676 (2003).
Takahashi, T. et al. Vα24+ natural killer T-cell responses against T-acute lymphoblastic leukaemia cells: implications for immunotherapy. Br. J. Haematol. 122, 231–239 (2003).
Fais, F. et al. CD1d is expressed on B-chronic lymphocytic leukemia cells and mediates α-galactosylceramide presentation to natural killer T lymphocytes. Int. J. Cancer 109, 402–411 (2004).
Emile, J. F., Fraitag, S., Leborgne, M., de Prost, Y. & Brousse, N. Langerhans' cell histiocytosis cells are activated Langerhans' cells. J. Pathol. 174, 71–76 (1994).
Fivenson, D. P. & Nickoloff, B. J. Distinctive dendritic cell subsets expressing factor XIIIa, CD1a, CD1b and CD1c in mycosis fungoides and psoriasis. J. Cutan. Pathol. 22, 223–228 (1995).
Villarroel Dorrego, M., Correnti, M., Delgado, R. & Tapia, F. J. Oral lichen planus: immunohistology of mucosal lesions. J. Oral Pathol. Med. 31, 410–414 (2002).
Hakomori, S. Cancer-associated glycosphingolipid antigens: their structure, organization, and function. Acta Anat. (Basel) 161, 79–90 (1998).
Sieling, P. A. et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J. Immunol. 165, 5338–5344 (2000).
De Libero, G. et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity (in the press).
Pomorski, T., Holthuis, J. C., Herrmann, A. & van Meer, G. Tracking down lipid flippases and their biological functions. J. Cell Sci. 117, 805–813 (2004).
Menon, A. K., Watkins, W. E. R. & Hrafnsdottir, S. Specific proteins are required to translocate phosphatidylcholine bidirectionally across the endoplasmic reticulum. Curr. Biol. 10, 241–252 (2000).
Zhou, Q. et al. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J. Biol. Chem. 272, 18240–18244 (1997).
Farge, E., Ojcius, D. M., Subtil, A. & Dautry-Varsat, A. Enhancement of endocytosis due to aminophospholipid transport across the plasma membrane of living cells. Am. J. Physiol. 276, C725–C733 (1999).
Smit, J. J. et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75, 451–462 (1993).
van Helvoort, A. et al. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 87, 507–517 (1996).
Broccardo, C., Luciani, M. & Chimini, G. The ABCA subclass of mammalian transporters. Biochim. Biophys. Acta 1461, 395–404 (1999).
Berge, K. E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290, 1771–1775 (2000).
Mosser, J. et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993).
Strauss, J. F., Kishida, T., Christenson, L. K., Fujimoto, T. & Hiroi, H. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol. Cell. Endocrinol. 202, 59–65 (2003).
Mukherjee, S. & Maxfield, F. R. Lipid and cholesterol trafficking in NPC. Biochim. Biophys. Acta 1685, 28–37 (2004).
Routt, S. M. & Bankaitis, V. A. Biological functions of phosphatidylinositol transfer proteins. Biochem. Cell Biol. 82, 254–262 (2004).
Riezman, H. & van Meer, G. Lipid pickup and delivery. Nature Cell Biol. 6, 15–16 (2004).
Albers, J. J. & Cheung, M. C. Emerging roles for phospholipid transfer protein in lipid and lipoprotein metabolism. Curr. Opin. Lipidol. 15, 255–260 (2004).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997). This was the first study to show that α-galactosylceramide stimulates CD1d-restricted iNKT cells. It also provided the first evidence that structural modifications in the sugar and lipid portions influence T-cell recognition.
Fischer, K. et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl Acad. Sci. USA 101, 10685–10690 (2004).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004). This paper reports isoglobotrihexosylceramide as the first mammalian glycosphingolipid to be identified as stimulating human and mouse iNKT cells. Although it remains to be formally proven that isoglobotrihexosylceramide is synthesized by human and mouse cells, this article provides a hint on the nature of the elusive endogenous iNKT antigens.
Acknowledgements
We thank all the members of our laboratory for their dedication and enthusiasm, T.-J. Resink and E. Palmer for discussions and reading of the manuscript, and A. Bendelac and M. Kronenberg for sharing data before publication. We apologize to those whose work has not been included. Our laboratory is supported by the University Hospital Basel, the Swiss National Science Foundation, the European Community and the Swiss Multiple Sclerosis Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
OMIM
FURTHER INFORMATION
Glossary
- MULTIPLE SCLEROSIS
-
(MS). A chronic inflammatory and demyelinating disease of the central nervous system. MS is characterized by an autoimmune response against components of myelin, which is thought to contribute to disease pathogenesis. Self glycolipids are autoantigens that are recognized by T cells in this disease.
- SIDEROPHORES
-
Numerous bacteria secrete these low molecular-weight compounds, which have a high affinity for iron and other metal ions. These molecules chelate metal ions and carry them into the cell through specific receptors. They represent bacterial virulence factors.
- CROSSPRIMING AND CROSSPRESENTATION
-
Mechanisms by which a professional antigen-presenting cell primes or activates a T cell that is specific for an antigen derived from a third cell type.
- EXOSOMES
-
Minute natural membrane vesicles that are secreted by various types of cells in the immune system.
- INVARIANT CHAIN
-
A non-polymorphic molecule that associates with MHC class II proteins. By occupying the antigen-binding cleft, the invariant chain stabilizes newly synthesized MHC class II molecules in the endoplasmic reticulum and directs the mature molecules to compartments in which binding with antigenic peptides occurs.
- LIPID-TRANSFER PROTEINS
-
(LTPs). Proteins with hydrophobic features (pockets and binding regions). LTPs bind lipids and facilitate the solubilization, transport and interaction of lipids with other proteins.
- TRANSLOCASE
-
A lipid translocase is an ATP-dependent flippase that facilitates the unidirectional movement of lipids from one membrane leaflet to the other. Translocases contribute to asymmetrical lipid distribution across the bilayer.
- PHAGOSOME
-
The functional definition of the organelle in which bacteria are internalized. Virulent bacteria inhibit the acidification of the phagosome and its fusion with lysosomes. This is an important escape mechanism that facilitates bacterial intracellular growth.
- FLIPPASE
-
A protein or protein complex that facilitates the energetically unfavourable movement of the polar head group of a phospholipid or glycosphingolipid through the hydrophobic interior of a membrane.
- SCRAMBLASE
-
An energy-independent bidirectional lipid flippase that, when activated by transient rises in intracellular Ca2+ levels, disrupts lipid asymmetry and facilitates the fast equilibration of lipids between the two membrane leaflets.
- COMPLEMENTARITY DETERMINING REGION 3
-
(CDR3). The antigen-receptor region (in T- and B-cell receptors) that interacts with the antigen, in which hypervariable sequences are located. CDR3 is partly encoded by the germline variable (V), diversity (D) and joining (J) regions of each receptor chain. Extensive diversity is generated during gene rearrangement by nucleotide trimming and/or template-independent nucleotide additions by terminal deoxynucleotidyl transferase.
Rights and permissions
About this article
Cite this article
De Libero, G., Mori, L. Recognition of lipid antigens by T cells. Nat Rev Immunol 5, 485–496 (2005). https://doi.org/10.1038/nri1631
Issue Date:
DOI: https://doi.org/10.1038/nri1631
This article is cited by
-
Cancer-associated adipocytes as immunomodulators in cancer
Biomarker Research (2021)
-
Tissue-specific functions of invariant natural killer T cells
Nature Reviews Immunology (2018)
-
The microbiota maintain homeostasis of liver-resident γδT-17 cells in a lipid antigen/CD1d-dependent manner
Nature Communications (2017)
-
Ligand binding to an Allergenic Lipid Transfer Protein Enhances Conformational Flexibility resulting in an Increase in Susceptibility to Gastroduodenal Proteolysis
Scientific Reports (2016)
-
Immunität gegen Mycobacterium tuberculosis
Der Internist (2016)