Key Points
-
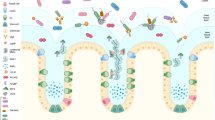
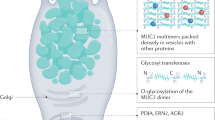
Mucins are highly O-glycosylated molecules that have gel-like properties. The mucin family consists of transmembrane mucins and gel-forming mucins. The transmembrane mucins cover the apical surfaces of the enterocytes and form the glycocalyx. The gel-forming mucins are secreted from goblet cells as large multimers that form the mucus skeleton and cover all epithelial surfaces.
-
Mucus in the small intestine forms a diffusion barrier where antimicrobial substances keep the epithelium free from microorganism. Mucus in the colon forms a dense inner mucus layer that bacteria are unable to penetrate, creating a bacteria-free zone at the epithelial surface.
-
Some, but not all, bacteria stimulate the formation of a functional mucus system with removable mucus in the small intestine and a stratified impenetrable inner mucus layer in colon.
-
Mucus in the intestine creates a niche for bacteria, with digestible glycans providing a stable energy source, but mucus also traps and removes bacteria. Bacteria in loose mucus are planktonic and less virulent.
-
The small intestinal goblet cells can sample luminal material during mucus secretion and transfer the antigens to lamina propria dendritic cells, something that also happens in the colon if bacterial numbers are decreased. This communication with the immune system has tolerogenic effects.
-
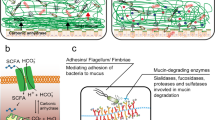
Intestinal pathogens have mechanisms that allow them to circumvent the mucus protection to reach the epithelium. These include good motility and secretion of enzymes that can degrade the otherwise protease-resistant mucins.
Abstract
A number of mechanisms ensure that the intestine is protected from pathogens and also against our own intestinal microbiota. The outermost of these is the secreted mucus, which entraps bacteria and prevents their translocation into the tissue. Mucus contains many immunomodulatory molecules and is largely produced by the goblet cells. These cells are highly responsive to the signals they receive from the immune system and are also able to deliver antigens from the lumen to dendritic cells in the lamina propria. In this Review, we will give a basic overview of mucus, mucins and goblet cells, and explain how each of these contributes to immune regulation in the intestine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ambort, D. et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl Acad. Sci. USA 109, 5645–5650 (2012).
Lang, T., Hansson, G. C. & Samuelsson, T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl Acad. Sci. USA 104, 16209–16214 (2007).
Hattrup, C. L. & Gendler, S. J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, 431–457 (2008).
Corfield, A. P. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 1850, 236–252 (2015).
Linden, S. K., Florin, T. H. & McGuckin, M. A. Mucin dynamics in intestinal bacterial infection. PLoS ONE 3, e3952 (2008).
Shibahara, H. et al. Pathobiological implications of mucin (MUC) expression in the outcome of small bowel cancer. PLoS ONE 9, e86111 (2014).
Thathiah, A. & Carson, D. D. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem. J. 382, 363–373 (2004).
Larsson, J. M. et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel. Dis. 17, 2299–2307 (2011).
Hasnain, S. Z., Gallagher, A. L., Grencis, R. K. & Thornton, D. J. A new role for mucins in immunity: insights from gastrointestinal nematode infection. Int. J. Biochem. Cell Biol. 45, 364–374 (2013).
Ridley, C. et al. Assembly of the respiratory mucin MUC5B: a new model for a gel-forming mucin. J. Biol. Chem. 289, 16409–16420 (2014).
Gum, J. R., Crawley, S. C., Hicks, J. W., Szymkowski, D. E. & Kim, Y. S. MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 291, 466–475 (2002).
Williams, S. J. et al. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 59, 4083–4089 (1999).
Pelaseyed, T., Gustafsson, J. K., Gustafsson, I. J., Ermund, A. & Hansson, G. C. Carbachol-induced MUC17 endocytosis is concomitant with NHE3 internalization and CFTR membrane recruitment in enterocytes. Am. J. Physiol. Cell Physiol. 305, C457–C467 (2013).
Sheng, Y. H. et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut 60, 1661–1670 (2011).
Gregorieff, A. et al. The Ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology 137, 1333–1345 (2009). This study shows that the transcription factor SPDEF is a major regulator of goblet and Paneth cell maturation and controls goblet cell-specific gene expression.
Park, S. W. et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl Acad. Sci. USA 106, 6950–6955 (2009).
Tsuru, A. et al. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc. Natl Acad. Sci. USA 110, 2864–2869 (2013).
Johansson, M. E. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS ONE 7, e41009 (2012).
Neutra, M. & Leblond, C. P. Synthesis of the carbohydrate of mucus in the Golgi complex as shown by electron microscope radioautography of goblet cells from rats injected with glucose-H3. J. Cell Biol. 30, 119–136 (1966).
Davis, C. W. & Dickey, B. F. Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol. 70, 487–512 (2008).
Specian, R. D. & Neutra, M. R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J. Cell Biol. 85, 626–640 (1980).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Tran, D. T., Masedunskas, A., Weigert, R. & Ten Hagen, K. G. Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat. Commun. 6, 10098 (2015).
Patel, K. K. et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 32, 3130–3144 (2013). This study shows that goblet cell secretion can be initiated via endocytosis and requires activation of autophagy proteins.
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
Birchenough, G. M. H., Nystrom, E. L. N., Johansson, M. E. V. & Hansson, G. C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6- dependent Muc2 secretion. Science 352, 1535–1542 (2016). This study describes a sentinel goblet cell that guards the colonic crypt opening and defends this region by stimulating mucus secretion.
Gustafsson, J. K. et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 209, 1263–1272 (2012).
Schutte, A. et al. Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc. Natl Acad. Sci. USA 111, 12396–12401 (2014).
Ermund, A., Schutte, A., Johansson, M. E., Gustafsson, J. K. & Hansson, G. C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G341–G347 (2013).
Hooper, L. V. & Macpherson, A. J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169 (2010).
Meyer-Hoffert, U. et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57, 764–771 (2008).
van der Waaij, L. A. et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm. Bowel. Dis. 11, 865–871 (2005).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Swidsinski, A., Loening-Baucke, V., Lochs, H. & Hale, L. P. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J. Gastroenterol. 11, 1131–1140 (2005).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008). This study describes the well-structured inner mucus layer of the two-layered mucus system that separates the colonic bacteria from the epithelium.
Kato, A. & Romero, M. F. Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 73, 261–281 (2011).
Swidsinski, A., Loening-Baucke, V., Verstraelen, H., Osowska, S. & Doerffel, Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 135, 568–579 (2008).
Li, H. et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun. 6, 8292 (2015).
Derrien, M., Collado, M. C., Ben-Amor, K., Salminen, S. & de Vos, W. M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 74, 1646–1648 (2008).
Robertson, B. R. et al. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int. J. Syst. Evol. Microbiol. 55, 1199–1204 (2005).
Macpherson, A. J., McCoy, K. D., Johansen, F. E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Jakobsson, H. E. et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 16, 164–177 (2015).
Slack, E., Balmer, M. L. & Macpherson, A. J. B cells as a critical node in the microbiota–host immune system network. Immunol. Rev. 260, 50–66 (2014).
Rogier, E. W., Frantz, A. L., Bruno, M. E. & Kaetzel, C. S. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathog. 3, 390–403 (2014).
Frantz, A. L. et al. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 5, 501–512 (2012).
Vaishnava, S. et al. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011).
Loonen, L. M. et al. REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 7, 939–947 (2014).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Earle, K. A. et al. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 18, 478–488 (2015).
Sommer, F. & Backhed, F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 8, 372–379 (2015).
Barr, J. J. et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl Acad. Sci. USA 110, 10771–10776 (2013).
Rodriguez-Pineiro, A. M. et al. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G348–G356 (2013).
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012). A report showing that small intestinal goblet cells take up lumenal material during secretion and deliver this material to lamina propria DCs.
Shan, M. et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013).
Kraehenbuhl, J. P. & Neutra, M. R. Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol. 16, 301–332 (2000).
Knoop, K. A., McDonald, K. G., McCrate, S., McDole, J. R. & Newberry, R. D. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 8, 198–210 (2015).
Knoop, K. A., McDonald, K. G., Kulkarni, D. H. & Newberry, R. D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65, 1100–1109 (2015).
Rosen, S. D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Khan, W. I. & Collins, S. M. Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. 26, 319–326 (2004).
Oeser, K., Schwartz, C. & Voehringer, D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol. 8, 672–682 (2015).
Finkelman, F. D. et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155 (2004).
Steenwinckel, V. et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J. Immunol. 182, 4737–4743 (2009).
von Moltke, J., Ji, M., Liang, H. E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature 529, 221–225 (2016).
Gerbe, F. et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016).
Rajavelu, P. et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Invest. 125, 2021–2031 (2015).
Turner, J. E., Stockinger, B. & Helmby, H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog. 9, e1003698 (2013).
Johansson, M. E. et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014). A study showing that colitic mice and patients with ulcerative colitis have a defect inner mucus layer that allows bacterial penetration.
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443 (2015).
El, A. S. et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 5, 567–579 (2012).
Johansson, M. E. et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18, 582–592 (2015).
Juge, N. Microbial adhesins to gastrointestinal mucus. Trends Microbiol. 20, 30–39 (2012).
Ley, R. E. et al. Evolution of mammals and their gut microbes. Science 320, 1647–1651 (2008).
Rawls, J. F., Mahowald, M. A., Ley, R. E. & Gordon, J. I. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127, 423–433 (2006). This study shows that host mechanisms are important for the selection of a host-specific microbiota.
Marcobal, A., Southwick, A. M., Earle, K. A. & Sonnenburg, J. L. A refined palate: bacterial consumption of host glycans in the gut. Glycobiology 23, 1038–1046 (2013).
Pudlo, N. A. et al. Symbiotic human gut bacteria with variable metabolic priorities for host mucosal glycans. mBio 6, e01282–15 (2015).
Sonnenburg, J. L. et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959 (2005).
Sonnenburg, E. D. et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016).
Caldara, M. et al. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol. 22, 2325–2330 (2012). This study showed that bacteria can swim in freshly prepared mucus and remain in a planktonic state.
Norkina, O., Burnett, T. G. & De Lisle, R. C. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect. Immun. 72, 6040–6049 (2004).
Velcich, A. et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295, 1726–1729 (2002). This study shows that mice lacking MUC2 have no protective mucus and develop cancer.
El, A. S. et al. Transient inflammatory-like state and microbial dysbiosis are pivotal in establishment of mucosal homeostasis during colonisation of germ-free mice. Benef. Microbes 5, 67–77 (2014).
Wrzosek, L. et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 11, 61 (2013).
Willing, B. P., Russell, S. L. & Finlay, B. B. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243 (2011).
Wlodarska, M. et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 79, 1536–1545 (2011).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Vijay-Kumar, M. et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117, 3909–3921 (2007).
Bhinder, G. et al. Intestinal epithelium-specific MyD88 signaling impacts host susceptibility to infectious colitis by promoting protective goblet cell and antimicrobial responses. Infect. Immun. 82, 3753–3763 (2014).
Hamer, H. M. et al. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 27, 104–119 (2008).
Gaudier, E. et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1168–G1174 (2004).
Artis, D. & Grencis, R. K. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 1, 252–264 (2008).
Stecher, B. et al. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72, 4138–4150 (2004).
Navarro-Garcia, F. et al. Pic, an autotransporter protein secreted by different pathogens in the Enterobacteriaceae family, is a potent mucus secretagogue. Infect. Immun. 78, 4101–4109 (2010).
Silva, A. J., Pham, K. & Benitez, J. A. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149, 1883–1891 (2003).
Nikitas, G. et al. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J. Exp. Med. 208, 2263–2277 (2011).
Bergstrom, K. S. et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6, e1000902 (2010).
Bhullar, K. et al. The serine protease autotransporter pic modulates citrobacter rodentium pathogenesis and its innate recognition by the host. Infect. Immun. 83, 2636–2650 (2015).
Lidell, M. E., Moncada, D. M., Chadee, K. & Hansson, G. C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc. Natl Acad. Sci. USA 103, 9298–9303 (2006).
van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Huang, E. Y. et al. Using corticosteroids to reshape the gut microbiome: implications for inflammatory bowel diseases. Inflamm. Bowel. Dis. 21, 963–972 (2015).
Liu, C. et al. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am. J. Physiol. Cell Physiol. 305, C121–C128 (2013).
Xiao, F. et al. Slc26a3 deficiency is associated with loss of colonic HCO3− secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol. (Oxf) 211, 161–175 (2013).
Fu, J. et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 121, 1657–1666 (2011).
Hickey, C. A. et al. Colitogenic bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe 17, 672–680 (2015).
Johansson, M. E. et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 5, e12238 (2010).
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Bertolotti, A. et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1β-deficient mice. J. Clin. Invest. 107, 585–593 (2001).
Bersudsky, M. et al. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut 63, 598–609 (2014).
Imaeda, H. et al. Interleukin-33 suppresses Notch ligand expression and prevents goblet cell depletion in dextran sulfate sodium-induced colitis. Int. J. Mol. Med. 28, 573–578 (2011).
Ito, R. et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 377, 12–16 (2008).
Grootjans, J. et al. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut 62, 250–258 (2013). This study proposed a conceptual model for how mucus secretion from the crypt can clear bacteria and reconstitute an inner mucus layer.
Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Das, I. et al. Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J. Exp. Med. 210, 1201–1216 (2013).
Wang, R. et al. Neutralizing IL-23 is superior to blocking IL-17 in suppressing intestinal inflammation in a spontaneous murine colitis model. Inflamm. Bowel Dis. 21, 973–984 (2015).
Pullan, R. D. et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35, 353–359 (1994).
Heylen, M. et al. Of worms, mice and man: an overview of experimental and clinical helminth-based therapy for inflammatory bowel disease. Pharmacol. Ther. 143, 153–167 (2014).
Audie, J. P. et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J. Histochem. Cytochem. 41, 1479–1485 (1993).
Weiss, A. A., Babyatsky, M. W., Ogata, S., Chen, A. & Itzkowitz, S. H. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J. Histochem. Cytochem. 44, 1161–1166 (1996).
Williams, S. J. et al. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 276, 18327–18336 (2001).
Chang, S. K. et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology 107, 28–36 (1994).
Ho, S. B. et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 55, 2681–2690 (1995).
Gruber, A. D. et al. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl− channel proteins. Genomics 54, 200–214 (1998).
Johansson, M. E., Thomsson, K. A. & Hansson, G. C. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J. Proteome. Res. 8, 3549–3557 (2009).
Madsen, J., Nielsen, O., Tornoe, I., Thim, L. & Holmskov, U. Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 55, 505–513 (2007).
Tateno, H. et al. Human ZG16p recognizes pathogenic fungi through non-self polyvalent mannose in the digestive system. Glycobiology 22, 210–220 (2012).
Kang, W. & Reid, K. B. DMBT1, a regulator of mucosal homeostasis through the linking of mucosal defense and regeneration? FEBS Lett. 540, 21–25 (2003).
Acknowledgements
The work of the authors was supported by the Swedish Research Council, the Swedish Cancer Foundation, the Knut and Alice Wallenberg Foundation, the IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (ALF), the National Institute of Allergy and Infectious Diseases (U01AI095473), the Hasselblad Foundation, and the Swedish Foundation for Strategic Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- SEA domain
-
(Sea urchin sperm protein, enterokinase, and agrin domain) A domain that is found on the outside of the membrane in several transmembrane mucins and autocatalytically cleaved during folding in the endoplasmic reticulum.
- Cystic fibrosis transmembrane conductance regulator
-
(CFTR). An ion channel that transports chloride and bicarbonate ions. Defects in this channel cause cystic fibrosis.
- Dextran sulfate sodium (DSS)-induced colitis
-
A model of colitis induced in rodents by the addition of DSS to drinking water; this causes the inner colon mucus layer to become penetrable to bacteria, disrupts the epithelial layer and leads to intestinal inflammation.
- SAM pointed domain-containing Ets transcription factor
-
(SPDEF). A transcription factor that is a master regulator of goblet cell lineage differentiation and maturation.
- Goblet cell theca
-
The cluster of large mucin-filled granulae that are typically observed in goblet cells.
- Segmented filamentous bacteria
-
Bacteria that infect the small intestine of the mouse, but not humans, attaching to the enterocyte membranes; they support T helper 17 cell responses in the mouse intestine.
Rights and permissions
About this article
Cite this article
Johansson, M., Hansson, G. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 16, 639–649 (2016). https://doi.org/10.1038/nri.2016.88
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.88
This article is cited by
-
Lacidophilin tablets alleviate constipation through regulation of intestinal microflora by promoting the colonization of Akkermansia sps
Scientific Reports (2024)
-
What if gastrointestinal complications in endurance athletes were gut injuries in response to a high consumption of ultra-processed foods? Please take care of your bugs if you want to improve endurance performance: a narrative review
European Journal of Applied Physiology (2024)
-
Increased Intestinal Permeability: An Avenue for the Development of Autoimmune Disease?
Exposure and Health (2024)
-
Pioneering gut health improvements in piglets with phytogenic feed additives
Applied Microbiology and Biotechnology (2024)
-
Changes in intestinal morphology, number of mucus-producing cells and expression of coronavirus receptors APN, DPP4, ACE2 and TMPRSS2 in pigs with aging
Veterinary Research (2023)