Key Points
-
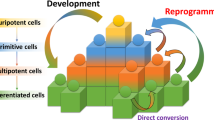
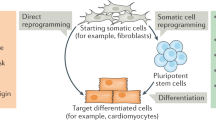
Direct reprogramming enables the generation of pluripotent stem-cell lines from almost any somatic tissue and mammalian species, thereby avoiding the ethical issues associated with human embryonic stem cells.
-
Although direct reprogramming is conceptually and technically simple, it is an extremely slow and inefficient process. It is influenced by several variables that affect its efficiency and reproducibility, and the quality of the resulting induced pluripotent stem cells (iPSCs).
-
Depending on the donor cell type, reprogramming is achieved with different efficiencies and kinetics. These differences are attributed to variations in the endogenous levels of certain reprogramming factors, differentiation status and/or intrinsic epigenetic states that are more amenable to reprogramming.
-
Different factors are able to promote reprogramming, including genes that are normally expressed in early development, factors that directly or indirectly affect cell proliferation, chromatin remodellers or microRNAs.
-
At present in the iPSC field, it is still difficult to unambiguously designate a reprogramming strategy that is fitting for all purposes. In each case, one will have to evaluate the most appropriate starting cell types, factors, culture conditions and delivery method.
-
Reprogramming methods can be divided into two classes, those involving the integration of exogenous genetic material and those involving no genetic modification of the donor cells. Among these methodologies, retroviral delivery of OCT4, SOX2, KLF4 and MYC (the OSKM set) into fibroblasts is still the most widely used.
-
Integrative reprogramming approaches generate heterogeneous iPSC lines, which could obscure comparative analysis between lines. The use of Cre-deletable vectors has partially solved this problem.
-
Among non-integrative reprogramming systems, the recently published RNA-based approach seems promising on the basis of the high efficiency it achieves. Although appealing, the high gene dosages of the reprogramming factors resulting from direct messenger RNA delivery may represent an oncogeneic risk owing to higher expression levels of MYC.
-
A crucial challenge in the iPSC field is to evaluate how these various methodologies affect the quality of iPSCs in terms of transcriptional signatures, epigenetic status, genomic integrity, stability, differentiation and tumorigenic potential. Whole-genome sequencing platforms will probably have an important role in the future in assessing the integrity of the genome of iPSCs and will certainly improve our understanding of the mechanism by which reprogramming occurs in a specific cell type.
Abstract
Pluripotent stem-cell lines can be obtained through the reprogramming of somatic cells from different tissues and species by ectopic expression of defined factors. In theory, these cells — known as induced pluripotent stem cells (iPSCs) — are suitable for various purposes, including disease modelling, autologous cell therapy, drug or toxicity screening and basic research. Recent methodological improvements are increasing the ease and efficiency of reprogramming, and reducing the genomic modifications required to complete the process. However, depending on the downstream applications, certain technologies have advantages over others. Here, we provide a comprehensive overview of the existing reprogramming approaches with the aim of providing readers with a better understanding of the reprogramming process and a basis for selecting the most suitable method for basic or clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638 (1981).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). Princeps paper describing the generation of a human blastocyst-derived, pluripotent cell lines relevant for human transplantation medicine.
Hochedlinger, K. & Jaenisch, R. Nuclear reprogramming and pluripotency. Nature 441, 1061–1067 (2006).
Eggan, K. et al. Mice cloned from olfactory sensory neurons. Nature 428, 44–49 (2004).
Hochedlinger, K. & Jaenisch, R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415, 1035–1038 (2002).
Li, J., Greco, V., Guasch, G., Fuchs, E. & Mombaerts, P. Mice cloned from skin cells. Proc. Natl Acad. Sci. USA 104, 2738–2743 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). The first paper describing the generation of iPSC lines from MEFs by overexpressing the Oct4, Sox2, Klf4 and Myc transcription factors.
Maherali, N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007).
Mikkelsen, T. S. et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 (2008).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007).
Aoi, T. et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321, 699–702 (2008).
Stadtfeld, M., Brennand, K. & Hochedlinger, K. Reprogramming of pancreatic β cells into induced pluripotent stem cells. Curr. Biol. 18, 890–894 (2008).
Eminli, S., Utikal, J., Arnold, K., Jaenisch, R. & Hochedlinger, K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells 26, 2467–2474 (2008).
Kim, J. B. et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454, 646–650 (2008).
Aasen, T. et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotech. 26, 1276–1284 (2008).
Giorgetti, A. et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell 5, 353–357 (2009). This paper describes the generation of iPSCs from cord blood CD133+ cells, which could facilitate the constitution of iPSC banks that represent a wide panel of relevant haplotypes that are useful for transplantation.
Loh, Y. H. et al. Generation of induced pluripotent stem cells from human blood. Blood 113, 5476–5479 (2009).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotech. 26, 101–106 (2008).
Wernig, M., Meissner, A., Cassady, J. P. & Jaenisch, R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2, 10–12 (2008).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Daley, G. Q. et al. Broader implications of defining standards for the pluripotency of iPSCs. Cell Stem Cell 4, 200–201; author reply 202 (2009).
Ellis, J. et al. Alternative induced pluripotent stem cell characterization criteria for in vitro applications. Cell Stem Cell 4, 198–199; author reply 202 (2009).
Maherali, N. & Hochedlinger, K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell 3, 595–605 (2008).
Kim, J. B. et al. Oct4-induced pluripotency in adult neural stem cells. Cell 136, 411–419 (2009).
Eminli, S. et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nature Genet. 41, 968–976 (2009). It is assumed that the level of differentiation of the donor cell population may represent a barrier to reprogramming. This article directly addresses this question by testing the reprogramming potential of mouse haematopoietic cells at different stages of differentiation.
Miura, K. et al. Variation in the safety of induced pluripotent stem cell lines. Nature Biotech. 27, 743–745 (2009). The first large-scale study evaluating the safety of iPSCs fora therapeutic context, by evaluating the teratoma-forming capacity of injected iPSC-derived secondary neurospheres generated from 36 mouse iPSC lines derived in 11 different ways.
Hanna, J. et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 (2009). An in-depth analysis of reprogramming in mouse cells, revealing the stochastic nature of this process and showing that almost all donor cells can give rise to iPSCs after continued culture and transcription-factor expression.
Zhao, Y. et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell 3, 475–479 (2008).
Tsubooka, N. et al. Roles of Sall4 in the generation of pluripotent stem cells from blastocysts and fibroblasts. Genes Cells 14, 683–694 (2009).
Park, I. H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Silva, J. et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 6, e253 (2008).
Mallanna, S. K. & Rizzino, A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev. Biol. 344, 16–25 (2010).
Wang, Y. et al. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nature Genet. 40, 1478–1483 (2008).
Judson, R. L., Babiarz, J. E., Venere, M. & Blelloch, R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nature Biotech. 27, 459–461 (2009).
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 23, 2134–2139 (2009).
Hong, H. et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature 460, 1132–1135 (2009).
Kawamura, T. et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460, 1140–1144 (2009). The inhibition of tumour suppressor genes such as p53 greatly improves reprogramming efficiency. This observation suggests that reprogramming per secan exert selective pressure on the pool of donor cells in which this pathway is impaired.
Li, H. et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460, 1136–1139 (2009).
Utikal, J. et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 (2009).
Huangfu, D. et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotech. 26, 795–797 (2008). The first of many of articles that show the ability of chemical compounds to increase reprogramming efficiency or completely replace defined factors used in reprogramming. This work opens the door to eventually generating iPSCs using only chemicals.
Li, W. et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells 27, 2992–3000 (2009).
Huangfu, D. et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotech. 26, 1269–1275 (2008).
Feldman, N. et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nature Cell Biol. 8, 188–194 (2006).
Shi, Y. et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 3, 568–574 (2008).
Mali, P. et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 28, 713–720 (2010).
Esteban, M. A. et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 (2010).
Chung, T. L. et al. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 28, 1848–1855 (2010).
Yoshida, Y., Takahashi, K., Okita, K., Ichisaka, T. & Yamanaka, S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5, 237–241 (2009).
Dravid, G. et al. Defining the role of Wnt/β-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells 23, 1489–1501 (2005).
Marson, A. et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 3, 132–135 (2008).
Okada, M., Oka, M. & Yoneda, Y. Effective culture conditions for the induction of pluripotent stem cells. Biochim. Biophys. Acta 1800, 956–963 (2010).
Kitamura, T. et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31, 1007–1014 (2003).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Takahashi, K., Okita, K., Nakagawa, M. & Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nature Protoc. 2, 3081–3089 (2007).
Hawley, R. G., Lieu, F. H., Fong, A. Z. & Hawley, T. S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1, 136–138 (1994).
Jahner, D. et al. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature 298, 623–628 (1982).
Stewart, C. L., Stuhlmann, H., Jahner, D. & Jaenisch, R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc. Natl Acad. Sci. USA 79, 4098–4102 (1982).
Hotta, A. & Ellis, J. Retroviral vector silencing during iPS cell induction: an epigenetic beacon that signals distinct pluripotent states. J. Cell. Biochem. 105, 940–948 (2008).
Blelloch, R., Venere, M., Yen, J. & Ramalho-Santos, M. Generation of induced pluripotent stem cells in the absence of drug selection. Cell Stem Cell 1, 245–247 (2007).
Yao, S. et al. Retrovirus silencing, variegation, extinction, and memory are controlled by a dynamic interplay of multiple epigenetic modifications. Mol. Ther. 10, 27–36 (2004).
Varas, F. et al. Fibroblast-derived induced pluripotent stem cells show no common retroviral vector insertions. Stem Cells 27, 300–306 (2009).
Hanna, J. et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318, 1920–1923 (2007). The first demonstration of the therapeutical potential of iPSCs in a humanized sickle-cell anaemia mouse model, highlighting the need to resolve the problems relating to the use of retroviruses and oncogenes in reprogramming for human therapy.
Brambrink, T. et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151–159 (2008).
Ryan, M. D. & Drew, J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 13, 928–933 (1994).
Ryan, M. D. & Flint, M. Virus-encoded proteinases of the picornavirus super-group. J. Gen. Virol. 78, 699–723 (1997).
Hasegawa, K., Cowan, A. B., Nakatsuji, N. & Suemori, H. Efficient multicistronic expression of a transgene in human embryonic stem cells. Stem Cells 25, 1707–1712 (2007).
Kaji, K. et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458, 771–775 (2009).
Rodriguez-Piza, I. et al. Reprogramming of human fibroblasts to induced pluripotent stem cells under xeno-free conditions. Stem Cells 28, 36–44 (2010).
Carey, B. W. et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc. Natl Acad. Sci. USA 106, 157–162 (2009).
Sommer, C. A. et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27, 543–549 (2009).
Sommer, C. A. et al. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells 28, 64–74 (2010).
Cary, L. C. et al. Transposon mutagenesis of baculoviruses: analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 172, 156–169 (1989).
Wang, W. et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc. Natl Acad. Sci. USA 105, 9290–9295 (2008).
Lacoste, A., Berenshteyn, F. & Brivanlou, A. H. An efficient and reversible transposable system for gene delivery and lineage-specific differentiation in human embryonic stem cells. Cell Stem Cell 5, 332–342 (2009).
Fraser, M. J., Cary, L., Boonvisudhi, K. & Wang, H. G. Assay for movement of Lepidopteran transposon IFP2 in insect cells using a baculovirus genome as a target DNA. Virology 211, 397–407 (1995).
Fraser, M. J., Ciszczon, T., Elick, T. & Bauser, C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol. Biol. 5, 141–151 (1996).
Wilson, M. H., Coates, C. J. & George, A. L. PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 15, 139–145 (2007).
Woltjen, K. et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458, 766–770 (2009).
Yusa, K., Rad, R., Takeda, J. & Bradley, A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nature Methods 6, 363–369 (2009).
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G. & Hochedlinger, K. Induced pluripotent stem cells generated without viral integration. Science 322, 945–949 (2008).
Sigal, S. H. et al. Evidence for a terminal differentiation process in the rat liver. Differentiation 59, 35–42 (1995).
Gupta, S. Hepatic polyploidy and liver growth control. Semin. Cancer Biol. 10, 161–171 (2000).
Zhou, W. & Freed, C. R. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells 27, 2667–2674 (2009).
Fusaki, N., Ban, H., Nishiyama, A., Saeki, K. & Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 85, 348–362 (2009).
Seki, T. et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 7, 11–14 (2010).
Tokusumi, T. et al. Recombinant Sendai viruses expressing different levels of a foreign reporter gene. Virus Res. 86, 33–38 (2002).
Li, H. O. et al. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74, 6564–6569 (2000).
Inoue, M. et al. Nontransmissible virus-like particle formation by F-deficient Sendai virus is temperature sensitive and reduced by mutations in M and HN proteins. J. Virol. 77, 3238–3246 (2003).
Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T. & Yamanaka, S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 322, 949–953 (2008).
González, F. et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc. Natl Acad. Sci. USA 106, 8918–8922 (2009).
Jia, F. et al. A nonviral minicircle vector for deriving human iPS cells. Nature Methods 7, 197–199 (2010).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Okita, K., Hong, H., Takahashi, K. & Yamanaka, S. Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nature Protoc. 5, 418–428 (2010).
Pollack, Y., Stein, R., Razin, A. & Cedar, H. Methylation of foreign DNA sequences in eukaryotic cells. Proc . Natl Acad . Sci . USA 77, 6463–6467 (1980).
Yates, J., Warren, N., Reisman, D. & Sugden, B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl Acad. Sci. USA 81, 3806–3810 (1984).
Yates, J. L., Warren, N. & Sugden, B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313, 812–815 (1985).
Chen, Z. Y., He, C. Y., Ehrhardt, A. & Kay, M. A. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol. Ther. 8, 495–500 (2003).
Chen, Z. Y., He, C. Y. & Kay, M. A. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum. Gene Ther. 16, 126–131 (2005).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
Inoue, M. et al. p53 protein transduction therapy: successful targeting and inhibition of the growth of the bladder cancer cells. Eur. Urol. 49, 161–168 (2006).
Michiue, H. et al. The NH2 terminus of influenza virus hemagglutinin-2 subunit peptides enhances the antitumor potency of polyarginine-mediated p53 protein transduction. J. Biol. Chem. 280, 8285–8289 (2005).
Wadia, J. S. & Dowdy, S. F. Protein transduction technology. Curr. Opin. Biotechnol. 13, 52–56 (2002).
Zhou, H. et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4, 381–384 (2009).
Kim, D. et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4, 472–476 (2009).
Stadtfeld, M., Maherali, N., Borkent, M. & Hochedlinger, K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nature Methods 7, 53–55 (2009).
Feng, B., Ng, J. H., Heng, J. C. & Ng, H. H. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell 4, 301–312 (2009).
Stadtfeld, M. et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 465, 175–181 (2010).
Boué, S., Paramonov, I., Barrero, M. J. & Izpisúa Belmonte, J. C. Analysis of human and mouse reprogramming of somatic cells to induced pluripotent stem cells. What is in the plate? PLoS ONE 5, e12664 (2010).
Acknowledgements
We apologize to those authors whose publications cannot be mentioned here owing to space constraints. F.G. was partially supported by a fellowship from the Swiss National Science Foundation. Work in the laboratory of J.C.I.B. was supported by grants from the G. Harold and Leila Y. Mathers Charitable Foundation, the Cellex Foundation, MICINN and Sanofi-Aventis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Relative efficiencies of reprogramming depending on factors and/or delivery modes in mouse embryonic fibroblasts (MEFs). (PDF 350 kb)
Supplementary information S2 (table)
Direct reprogramming of diverse mouse cell types. (PDF 393 kb)
Supplementary information S3 (table)
Direct reprogramming of diverse human cell types. (PDF 541 kb)
Supplementary information S4 (table)
Disease iPS lines (PDF 316 kb)
Related links
Glossary
- Inner cell mass
-
(ICM). In mammals, a cluster of pluripotent cells found inside the blastocyst that give rise to all the cells of the body of the embryo proper. Embryonic stem cells, which are derived from ICM cells, are the closest in vitro counterpart of ICM cells.
- OCT4
-
(Also known as POU5F1). A POU homeodomain transcription factor that has a crucial role in early embryonic development and is necessary for the maintenance of embryonic stem cell pluripotency.
- SOX2
-
Transcription factor of the SRY-related HMG-box family involved in the regulation of embryonic development and in the determination of cell fate. SOX2 is required to maintain self-renewal of undifferentiated embryonic and neural stem cells.
- KLF4
-
A member of the Krüppel-like family of zinc finger transcription factors that is involved in cell proliferation, differentiation and survival. KLF4 has both transcriptional activation and repression domains.
- MYC
-
(Also known as c-MYC). MYC is among the most frequently dysregulated oncogenes in human cancer. This transcription factor controls the expression of hundreds of target genes, many of which are also oncogenes or tumour suppressors, and have roles in cell proliferation and the cell cycle.
- OSKM
-
Combination of the OCT4, SOX2, KLF4 and MYC transcription factors, also known as the 'Yamanaka factors'. This was the first combination that was reported to reprogramme somatic cells into a pluripotent state.
- Cord blood
-
The fraction of blood remaining in the placenta and the umbilical cord after childbirth. Cord blood is a rich source of haematopoietic stem cells, which have been used extensively for transplantation in the treatment of diseases such as leukaemia and other cancers.
- CD133+ cells
-
Cells expressing the CD133 antigen, a 97 kDa glycoprotein composed of five transmembrane domains. This cell-surface marker is expressed by immature haematopoietic stem/progenitor cells but not their mature counterparts.
- NANOG
-
A homeobox transcription factor expressed in undifferentiated cells, including fetal gonads (ovary and testis), inner cell mass and embryonic stem cells. NANOG expression in the inner cell mass prevents this from differentiating into extra-embryonic endoderm and trophectoderm.
- Alkaline phosphatase
-
A hydrolase enzyme responsible for dephosphorylating molecules such as nucleotides, proteins and alkaloids under alkaline conditions. It is often used as marker of pluripotency.
- p53
-
A tumour suppressor that responds to diverse cellular stresses by regulating genes involved in cell-cycle arrest, apoptosis, senescence, DNA repair and changes in metabolism. Downregulation of p53 improves reprogramming efficiency.
- Moloney murine leukaemia virus
-
(MMLV). A retrovirus composed of an ssRNA genome replicating through a DNA intermediate that integrates into the host genome. MMLV infects only actively dividing cells.
- Transfection
-
Delivery of nucleic acids (plasmid DNA, linear DNA or RNA) into cells by a non-viral method. Common transfection methods include calcium phosphate treatment, electroporation, nucelofection and the use of cationic lipid vehicles.
- Lentiviruses
-
A genus of retroviruses with long incubation periods that cause chronic, progressive and usually fatal diseases, such as HIV in humans. They are the only retroviruses that are able to replicate in non-dividing cells.
- Tet-inducible
-
An inducible promoter system based on the tetracycline operon, which is present in a variety of vectors. In Tet-OFF vectors, gene expression is turned on when tetracycline or doxycycline is removed from the culture medium, whereas Tet-ON systems are induced only when doxycycline is added.
- Cre
-
Cre is a 38-kDa type I topoisomerase protein from bacteriophage P1 that catalyses site-specific intramolecular (excision or inversion) and intermolecular (integration) recombination between loxP sites. The loxP site consists of two 13bp inverted repeats separated by an 8bp asymmetric spacer region.
- Polycistronic
-
A transcription unit made up of several open reading frames, resulting in the translation of separate proteins. Internal ribosome entry site or 2A-peptide sequences allow such multigene expression constructs to be engineered.
- piggyBac
-
(PB). A TTAA-specific transposon, originally described in the order Lepidoptera. This mobile genetic element stably transfers exogenous DNA into a variety of cells. The PB system is composed of a donor plasmid, co-transfected with a helper plasmid expressing the transposase. Once integrated, PBs can be precisely deleted upon remobilization by the transposase.
- FLP
-
The FLP recombination system, derived from the 2μ plasmid of Saccharomyces cerevisiae, mediates site-specific intramolecular (excision or inversion) and intermolecular (integration) recombination between FRT sites. The FRT site consists of two 13bp inverted repeats separated by an 8-bp asymmetric spacer.
- Adenoviral vector
-
A vector based on adenoviruses, which are medium-sized viruses with a double-stranded linear DNA genome. Recombinant adenoviral vectors allow transient, high-level expression of exogenous genes without integrating into the host genome.
- Sendai viral vector
-
A vector based on a negative sense, ssRNA paramyxovirus. F-deficient Sendai viral vectors replicate in the form of negative-sense ssRNA in the cytoplasm of infected cells, allowing the transfer of foreign genetic material.
- Episome
-
An extrachromosomal DNA element that can replicate within a cell independently of the chromosome. Commonly used episomal vectors (also referred to as plasmids) contain an origin of replication and an antibiotic resistance cassette, allowing propagation in bacteria.
- LIN28
-
Human LIN28 is a cytoplasmic RNA-binding protein containing an amino-terminal cold-shock domain and two carboxy-terminal CCHC zinc finger domains. It is expressed in various undifferentiated embryonic cell types, as well as adult cardiac and skeletal muscle cells. The expression of LIN28 is regulated by microRNAs.
- PhiC31
-
The PhiC31 (ΦC31) integrase from bacteriophage PhiC31 is a serine-type site-specific recombinase that mediates the recombination between the heterotypic target sites attB and attP. Unlike Cre or FLP, this system allows irreversible deletion, inversion or integration between its target sites.
- B18R protein
-
A vaccinia virus decoy receptor for type I interferons. In some cell types it increases cell viability after RNA transfection.
Rights and permissions
About this article
Cite this article
González, F., Boué, S. & Belmonte, J. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nat Rev Genet 12, 231–242 (2011). https://doi.org/10.1038/nrg2937
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2937
This article is cited by
-
Correlation Between Alkaline Phosphatase Expression and Sox2, Oct4, and Nanog Genes in Spermatogonial and ES-Like Cells
Regenerative Engineering and Translational Medicine (2023)
-
Advances in RNA Viral Vector Technology to Reprogram Somatic Cells: The Paramyxovirus Wave
Molecular Diagnosis & Therapy (2022)
-
Induced Pluripotent Stem Cell-Derived Corneal Cells: Current Status and Application
Stem Cell Reviews and Reports (2022)
-
A Concise Review on Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Personalized Regenerative Medicine
Stem Cell Reviews and Reports (2021)
-
Treating primary immunodeficiencies with defects in NK cells: from stem cell therapy to gene editing
Stem Cell Research & Therapy (2020)