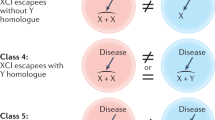
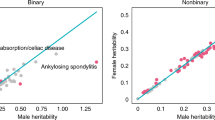
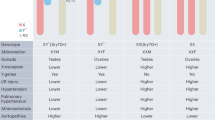
Key Points
-
Nearly all human diseases are sexually dimorphic with respect to prevalence, age of onset, severity or disease course. Sex-specific differences in physiology, behaviour or anatomy might contribute to some of the differences in disease risk, but genetics also plays a part.
-
Gene expression patterns differ between males and females of all species examined, not only for genes on the sex chromosomes, but also for genes on the autosomes.
-
Genes with sex-biased gene expression evolve rapidly at the protein-coding level, whereas differences in gene regulation are often highly conserved.
-
Differences in gene expression between the sexes probably contribute to sexual dimorphism in disease risk and course.
-
Studies of disease-associated quantitative traits in humans suggest that many have a sex-specific genetic architecture, with estimates of heritability differing between males and females.
-
Genotype-by-sex interactions are common in model organisms, indicating that genotype-specific effects differ between males and females. Recent examples of genotype-by-sex interactions on disease risk suggest that such effects might be common in humans as well.
-
Genetic linkage and association studies that do not consider sex-specific genotype effects could miss a significant proportion of genes contributing to risk for complex diseases.
Abstract
Sexual dimorphism in anatomical, physiological and behavioural traits are characteristics of many vertebrate species. In humans, sexual dimorphism is also observed in the prevalence, course and severity of many common diseases, including cardiovascular diseases, autoimmune diseases and asthma. Although sex differences in the endocrine and immune systems probably contribute to these observations, recent studies suggest that sex-specific genetic architecture also influences human phenotypes, including reproductive, physiological and disease traits. It is likely that an underlying mechanism is differential gene regulation in males and females, particularly in sex steroid-responsive genes. Genetic studies that ignore sex-specific effects in their design and interpretation could fail to identify a significant proportion of the genes that contribute to risk for complex diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alonso, L. C. & Rosenfield, R. L. Oestrogens and puberty. Best Pract. Res. Clin. Endocrinol. Metab. 16, 13–30 (2002).
Carrel, L. & Willard, H. F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404 (2005).
Korstanje, R. et al. Influence of sex and diet on quantitative trait loci for HDL cholesterol levels in an SM/J by NZB/BlNJ intercross population. J. Lipid Res. 45, 881–888 (2004).
Mackay, T. F. The genetic architecture of quantitative traits: lessons from Drosophila. Curr. Opin. Genet. Dev. 14, 253–257 (2004). A classic review of gene–environment (including genotype–sex) interactions in Drosophila.
Ueno, T. et al. Rat model of familial combined hyperlipidemia as a result of comparative mapping. Physiol. Genomics 17, 38–47 (2004).
Choi, B. G. & McLaughlin, M. A. Why men's hearts break: cardiovascular effects of sex steroids. Endocrinol. Metab. Clin. North Am. 36, 365–377 (2007).
Postma, D. S. Gender differences in asthma development and progression. Gend. Med. 4 (Suppl. B), S133–S146 (2007).
Lockshin, M. D. Sex differences in autoimmune disease. Lupus 15, 753–756 (2006).
Harper, P. S. Practical Genetic Counseling 5th edn (Reed Educational and Professional Publishing, Oxford, 1998).
Gater, R. et al. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch. Gen. Psychiatry 55, 405–413 (1998).
Andersen, K. et al. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology 53, 1992–1997 (1999).
Aleman, A., Kahn, R. S. & Selten, J. P. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch. Gen. Psychiatry 60, 565–571 (2003).
Wooten, G. F., Currie, L. J., Bovbjerg, V. E., Lee, J. K. & Patrie, J. Are men at greater risk for Parkinson's disease than women? J. Neurol. Neurosurg. Psychiatry 75, 637–639 (2004).
Matanoski, G., Tao, X. G., Almon, L., Adade, A. A. & Davies-Cole, J. O. Demographics and tumor characteristics of colorectal cancers in the United States, 1998–2001. Cancer 107, 1112–1120 (2006).
Patsopoulos, N. A., Tatsioni, A. & Ioannidis, J. P. Claims of sex differences: an empirical assessment in genetic associations. JAMA 298, 880–893 (2007). A comprehensive review and critique of the evidence for sex-specific genetic effects on risk for common diseases.
Barrett-Connor, E. Commentary: masculinity, femininity and heart disease. Int. J. Epidemiol. 36, 621–622 (2007).
Uekert, S. J. et al. Sex-related differences in immune development and the expression of atopy in early childhood. J. Allergy Clin. Immunol. 118, 1375–1381 (2006).
Whitacre, C. C., Reingold, S. C. & O'Looney, P. A. A gender gap in autoimmunity. Science 283, 1277–1278 (1999).
Straub, R. H. The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574 (2007).
Dobyns, W. B. et al. Inheritance of most X-linked traits is not dominant or recessive, just X-linked. Am. J. Med. Genet. A 129A, 136–143 (2004).
Lahn, B. T. & Page, D. C. Functional coherence of the human Y chromosome. Science 278, 675–680 (1997).
Lange, J., Skaletsky, H., Bell, G. W. & Page, D. C. MSY Breakpoint Mapper, a database of sequence-tagged sites useful in defining naturally occurring deletions in the human Y chromosome. Nucleic Acids Res. 36, D809–814 (2008).
Reinius, B. et al. An evolutionarily conserved sexual signature in the primate brain. PLoS Genet. 4, e1000100 (2008). An elegant demonstration of the conserved evolution of sexual dimorphism in gene expression patterns in the brain of primates.
Rinn, J. L. & Snyder, M. Sexual dimorphism in mammalian gene expression. Trends Genet. 21, 298–305 (2005). A modern overview of the evolution of sexual dimorphism in gene expression.
Yang, X. et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16, 995–1004 (2006).
Ellegren, H. & Parsch, J. The evolution of sex-biased genes and sex-biased gene expression. Nature Rev. Genet. 8, 689–698 (2007). A comprehensive review of sexual dimorphism in the regulatory genome.
Reinke, V., Gil, I. S., Ward, S. & Kazmer, K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131, 311–323 (2004).
Ranz, J. M., Castillo-Davis, C. I., Meiklejohn, C. D. & Hartl, D. L. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300, 1742–1745 (2003). One of the first genome-wide characterizations of sex-biased gene expression patterns in more than one species of Drosophila.
Baker, D. A., Meadows, L. A., Wang, J., Dow, J. A. & Russell, S. Variable sexually dimorphic gene expression in laboratory strains of Drosophila melanogaster. BMC Genomics 8, 454 (2007).
Santos, E. M., Kille, P., Workman, V. L., Paull, G. C. & Tyler, C. R. Sexually dimorphic gene expression in the brains of mature zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 149, 314–324 (2008).
Nishida, Y., Yoshioka, M. & St-Amand, J. Sexually dimorphic gene expression in the hypothalamus, pituitary gland, and cortex. Genomics 85, 679–687 (2005).
Zhang, Y., Sturgill, D., Parisi, M., Kumar, S. & Oliver, B. Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450, 233–237 (2007).
Sartori-Valinotti, J. C., Iliescu, R., Fortepiani, L. A., Yanes, L. L. & Reckelhoff, J. F. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 34, 938–945 (2007).
Zammaretti, F., Panzica, G. & Eva, C. Sex-dependent regulation of hypothalamic neuropeptide Y-Y1 receptor gene expression in moderate/high fat, high-energy diet-fed mice. J. Physiol. 583, 445–454 (2007).
Bhasin, J. M. et al. Sex specific gene regulation and expression QTLs in mouse macrophages from a strain intercross. PLoS ONE 3, e1435 (2008). The first explicit study of genetic variation that affects sex-specific variation in gene expression and of sex-specific eQTLs in a mammalian species.
Angelopoulou, R., Lavranos, G. & Manolakou, P. Establishing sexual dimorphism in humans. Coll. Antropol. 30, 653–658 (2006).
Visscher, P. M., Hill, W. G. & Wray, N. R. Heritability in the genomics era — concepts and misconceptions. Nature Rev. Genet. 9, 255–266 (2008). An excellent review of the use and misuse of the concept of heritability and its role in modern genetic studies.
Shea, M. K. et al. Genetic and non-genetic correlates of vitamins K and D. Eur. J. Clin. Nutr. 21 Nov 2007 (doi: 10.1038/sj.ejcn.1602959).
Santamaria, A. et al. Quantitative trait locus on chromosome 12q14.1 influences variation in plasma plasminogen levels in the San Antonio Family Heart Study. Hum. Biol. 79, 515–523 (2007).
de Simone, G. et al. Assessment of the interaction of heritability of volume load and left ventricular mass: the HyperGEN offspring study. J. Hypertens. 25, 1397–1402 (2007).
Pan, L., Ober, C. & Abney, M. Heritability estimation of sex-specific effects on human quantitative traits. Genet. Epidemiol. 31, 338–347 (2007).
Weiss, L. A., Pan, L., Abney, M. & Ober, C. The sex-specific genetic architecture of quantitative traits in humans. Nature Genet. 38, 218–222 (2006).
Ober, C., Abney, M. & McPeek, M. S. The genetic dissection of complex traits in a founder population. Am. J. Hum. Genet. 69, 1068–1079 (2001).
Abney, M., McPeek, M. S. & Ober, C. Heritabilities of quantitative traits in a founder population. Am. J. Hum. Genet. 68, 1302–1307 (2001).
Seda, O. et al. Systematic, genome-wide, sex-specific linkage of cardiovascular traits in French Canadians. Hypertension 51, 1156–1162 (2008).
Karasik, D. & Ferrari, S. L. Contribution of gender-specific genetic factors to osteoporosis risk. Ann. Hum. Genet. 72, 696–714 (2008).
Wang, C. et al. A computational model for sex-specific genetic architecture of complex traits in humans: implications for mapping pain sensitivity. Mol. Pain 4, 13 (2008).
Brookes, S. T. et al. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J. Clin. Epidemiol. 57, 229–236 (2004).
Higaki, J. et al. Deletion allele of angiotensin-converting enzyme gene increases risk of essential hypertension in Japanese men: the Suita Study. Circulation 101, 2060–2065 (2000).
O'Donnell, C. J. et al. Evidence for association and genetic linkage of the angiotensin-converting enzyme locus with hypertension and blood pressure in men but not women in the Framingham Heart Study. Circulation 97, 1766–1772 (1998).
Stankovic, A., Zivkovic, M. & Alavantic, D. Angiotensin I-converting enzyme gene polymorphism in a Serbian population: a gender-specific association with hypertension. Scand. J. Clin. Lab. Invest. 62, 469–475 (2002).
Fornage, M. et al. Variation in the region of the angiotensin-converting enzyme gene influences interindividual differences in blood pressure levels in young white males. Circulation 97, 1773–1779 (1998).
Weiss, L. A., Abney, M., Cook, E. H., Jr & Ober, C. Sex-specific genetic architecture of whole blood serotonin levels. Am. J. Hum. Genet. 76, 33–41 (2005).
Anholt, R. R. & Mackay, T. F. Quantitative genetic analyses of complex behaviours in Drosophila. Nature Rev. Genet. 5, 838–849 (2004).
Mackay, T. F. & Anholt, R. R. Of flies and man: Drosophila as a model for human complex traits. Annu. Rev. Genomics Hum. Genet. 7, 339–367 (2006).
Melo, J. A., Shendure, J., Pociask, K. & Silver, L. M. Identification of sex-specific quantitative trait loci controlling alcohol preference in C57BL/6 mice. Nature Genet. 13, 147–153 (1996).
Peirce, J. L., Derr, R., Shendure, J., Kolata, T. & Silver, L. M. A major influence of sex-specific loci on alcohol preference in C57Bl/6 and DBA/2 inbred mice. Mamm. Genome 9, 942–948 (1998).
Athirakul, K. et al. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 20 Aug 2008 (doi: fj.08-114413v1).
Ponder, C. A., Munoz, M., Gilliam, T. C. & Palmer, A. A. Genetic architecture of fear conditioning in chromosome substitution strains: relationship to measures of innate (unlearned) anxiety-like behavior. Mamm. Genome 18, 221–228 (2007).
Mattson, D. L. et al. Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am. J. Physiol. Renal Physiol. 293, F1905–F1914 (2007).
Tu, K., Chen, Z. & Lipscombe, L. L. Prevalence and incidence of hypertension from 1995 to 2005: a population-based study. CMAJ 178, 1429–1435 (2008).
Kearney, P. M. et al. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005).
Burt, V. L. et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 25, 305–313 (1995).
Martins, D., Nelson, K., Pan, D., Tareen, N. & Norris, K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J. Gend. Specif. Med. 4, 10–13, 20 (2001).
Kato, N. et al. Comprehensive analysis of the renin-angiotensin gene polymorphisms with relation to hypertension in the Japanese. J. Hypertens. 18, 1025–1032 (2000).
Rigat, B. et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 86, 1343–1346 (1990).
Krege, J. H. et al. Male–female differences in fertility and blood pressure in ACE-deficient mice. Nature 375, 146–148 (1995).
Leung, A. & Chue, P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr. Scand. Suppl. 401, 3–38 (2000).
McGrath, J. et al. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2, 13 (2004).
Cardno, A. G. & Gottesman, II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am. J. Med. Genet. 97, 12–17 (2000).
Shifman, S. et al. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 4, e28 (2008).
Wedenoja, J. et al. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol. Psychiatry 13, 673–684 (2008).
Hong, S. E. et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nature Genet. 26, 93–96 (2000).
Eastwood, S. L. & Harrison, P. J. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol. Psychiatry 8, 769, 821–731 (2003).
Hassold, T. & Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Rev. Genet. 2, 280–291 (2001).
Baker, B. S., Carpenter, A. T., Esposito, M. S., Esposito, R. E. & Sandler, L. The genetic control of meiosis. Annu. Rev. Genet. 10, 53–134 (1976).
Coop, G. & Przeworski, M. An evolutionary view of human recombination. Nature Rev. Genet. 8, 23–34 (2007).
Kong, A. et al. Sequence variants in the RNF212 gene associate with genome-wide recombination rate. Science 319, 1398–1401 (2008).
Coop, G., Wen, X., Ober, C., Pritchard, J. K. & Przeworski, M. High-resolution mapping of crossovers reveals extensive variation in fine-scale recombination patterns among humans. Science 319, 1395–1398 (2008).
Broman, K. W., Murray, J. C., Sheffield, V. C., White, R. L. & Weber, J. L. Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am. J. Hum. Genet. 63, 861–869 (1998).
Cheung, V. G., Burdick, J. T., Hirschmann, D. & Morley, M. Polymorphic variation in human meiotic recombination. Am. J. Hum. Genet. 80, 526–530 (2007).
Martinez, F. D. CD14, endotoxin, and asthma risk: actions and interactions. Proc. Am. Thorac. Soc. 4, 221–225 (2007).
Zambelli-Weiner, A. et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J. Allergy Clin. Immunol. 115, 1203–1209 (2005).
Simpson, A. et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am. J. Respir. Crit. Care Med. 174, 386–392 (2006).
Berchtold, N. C. et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl Acad. Sci. USA 105, 15605–15610 (2008).
Gupta, V. & Singh, S. M. Sex dimorphism in antitumor response of chemotherapeutic drug cisplatin in a murine host-bearing a T-cell lymphoma. Anticancer Drugs 19, 583–592 (2008).
Jackson, A., Stephens, D. & Duka, T. Gender differences in response to lorazepam in a human drug discrimination study. J. Psychopharmacol. 19, 614–619 (2005).
Klein, W. Gender differences in clinical trials in coronary heart disease: response to drug therapy. Eur. Heart J. 17, 1786–1790 (1996).
Dixon, A. L. et al. A genome-wide association study of global gene expression. Nature Genet. 39, 1202–1207 (2007).
Stranger, B. E. et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 315, 848–853 (2007).
Zhang, W. et al. Evaluation of genetic variation contributing to differences in gene expression between populations. Am. J. Hum. Genet. 82, 631–640 (2008).
Morley, M. et al. Genetic analysis of genome-wide variation in human gene expression. Nature 430, 743–747 (2004).
Storey, J. D. A direct approach to false discovery rates. J. Royal Stat. Soc. B 64, 479–198 (2002).
Gilad, Y., Rifkin, S. A. & Pritchard, J. K. Revealing the architecture of gene regulation: the promise of eQTL studies. Trends Genet. 24, 408–415 (2008).
Darwin, C. On the Origins of Species by Means of Natural Selection (John Murray, London, 1859).
Cunningham, J. T. Sexual Dimorphism in the Animal Kingdom (Adam and Charles Black, London, 1900).
Kimura, K. I., Ote, M., Tazawa, T. & Yamamoto, D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229–233 (2005).
Stockinger, P., Kvitsiani, D., Rotkopf, S., Tirian, L. & Dickson, B. J. Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 (2005).
Kimchi, T., Xu, J. & Dulac, C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014 (2007).
Plavcan, J. M. Sexual dimorphism in primate evolution. Yearb. Phys. Anthropol. 44, 25–53 (2001).
Andersson, M. Sexual Selection (Princeton Univ. Press, New Jersey, 1994). Comprehensive review and synthesis of essential topics in sexual selection, providing insight into the evolution of sex differences in nature and the role of selection and constraint in the development of secondary sexual traits.
Geary, D. C. Male, Female: The Evolution of Human Sex Differences (American Psychological Association, Washington DC, 1998).
Wizeman, T. M. & Pardue, M.-L. (eds) Exploring the Biological Contributions to Human Health: Does Sex Matter? (National Academy Press, Washington DC, 2001).
Badyaev, A. V. & Hill, G. E. Avian sexual dichromatism in relation to phylogeny and ecology. Ann. Rev. Ecol. Evol. Syst. 34, 27–49 (2003).
Lande, R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 (1980). A classic study that uses quantitative population genetic models to show that genetic correlations influence the expression and evolution of sexually dimorphic traits.
Rice, W. R. & Chippindale, A. K. Intersexual ontogenetic conflict. J. Evol. Biol. 14, 685–693 (2001).
Nelson, J. L. Microchimerism in human health and disease. Autoimmunity 36, 5–9 (2003).
Lambert, N. C. et al. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum. 50, 906–914 (2004).
Maloney, S. et al. Microchimerism of maternal origin persists into adult life. J. Clin. Invest. 104, 41–47 (1999).
Reed, A. M., Picornell, Y. J., Harwood, A. & Kredich, D. W. Chimerism in children with juvenile dermatomyositis. Lancet 356, 2156–2157 (2000).
Reed, A. M., McNallan, K., Wettstein, P., Vehe, R. & Ober, C. Does HLA-dependent chimerism underlie the pathogenesis of juvenile dermatomyositis? J. Immunol. 172, 5041–5046 (2004).
Yan, Z. et al. Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am. J. Med. 118, 899–906 (2005).
Loubiere, L. S. et al. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab. Invest. 86, 1185–1192 (2006).
Stevens, A. M., Hermes, H. M., Rutledge, J. C., Buyon, J. P. & Nelson, J. L. Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet 362, 1617–1623 (2003).
Stevens, A. M. et al. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab. Invest. 84, 1603–1609 (2004).
Khosrotehrani, K., Johnson, K. L., Cha, D. H., Salomon, R. N. & Bianchi, D. W. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA 292, 75–80 (2004).
Nelson, J. L. et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet 351, 559–562 (1998).
Johnson, K. L. et al. Fetal cell microchimerism in tissue from multiple sites in women with systemic sclerosis. Arthritis Rheum. 44, 1848–1854 (2001).
Ando, T., Imaizumi, M., Graves, P. N., Unger, P. & Davies, T. F. Intrathyroidal fetal microchimerism in Graves' disease. J. Clin. Endocrinol. Metab. 87, 3315–3320 (2002).
Nelson, J. L. et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc. Natl Acad. Sci. USA 104, 1637–1642 (2007).
Klintschar, M., Schwaiger, P., Mannweiler, S., Regauer, S. & Kleiber, M. Evidence of fetal microchimerism in Hashimoto's thyroiditis. J. Clin. Endocrinol. Metab. 86, 2494–2498 (2001).
Artlett, C. M. et al. Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Childhood Myositis Heterogeneity Collaborative Group. Lancet 356, 2155–2156 (2000).
Miyashita, Y., Ono, M., Ono, M., Ueki, H. & Kurasawa, K. Y chromosome microchimerism in rheumatic autoimmune disease. Ann. Rheum. Dis. 59, 655–656 (2000).
Badenhoop, K. Intrathyroidal microchimerism in Graves' disease or Hashimoto's thyroiditis: regulation of tolerance or alloimmunity by fetal-maternal immune interactions? Eur. J. Endocrinol. 150, 421–423 (2004).
Nelson, J. L. Maternal–fetal immunology and autoimmune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 39, 191–194 (1996).
Gammill, H. S. & Nelson, J. L. Naturally acquired microchimerism. Int. J. Dev. Biol. (in the press).
Reik, W. & Walter, J. Genomic imprinting: parental influence on the genome. Nature Rev. Genet. 2, 21–32 (2001).
Falls, J. G., Pulford, D. J., Wylie, A. A. & Jirtle, R. L. Genomic imprinting: implications for human disease. Am. J. Pathol. 154, 635–647 (1999).
Wilkins, J. F. & Haig, D. What good is genomic imprinting: the function of parent-specific gene expression. Nature Rev. Genet. 4, 359–368 (2003).
Morison, I. M., Ramsay, J. P. & Spencer, H. G. A census of mammalian imprinting. Trends Genet. 21, 457–465 (2005).
Bunzel, R. et al. Polymorphic imprinting of the serotonin-2A (5-HT2A) receptor gene in human adult brain. Brain Res. Mol. Brain Res. 59, 90–92 (1998).
Giannoukakis, N., Deal, C., Paquette, J., Kukuvitis, A. & Polychronakos, C. Polymorphic functional imprinting of the human IGF2 gene among individuals, in blood cells, is associated with H19 expression. Biochem. Biophys. Res. Commun. 220, 1014–1019 (1996).
Wolf, J. B., Cheverud, J. M., Roseman, C. & Hager, R. Genome-wide analysis reveals a complex pattern of genomic imprinting in mice. PLoS Genet. 4, e1000091 (2008).
Kaufman, J. M. & Vermeulen, A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 26, 833–876 (2005).
Khosla, S. et al. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J. Clin. Endocrinol. Metab. 83, 2266–2274 (1998).
Winters, S. J., Talbott, E., Guzick, D. S., Zborowski, J. & McHugh, K. P. Serum testosterone levels decrease in middle age in women with the polycystic ovary syndrome. Fertil. Steril. 73, 724–729 (2000).
Cooper, G. S. & Stroehla, B. C. The epidemiology of autoimmune diseases. Autoimmun. Rev. 2, 119–125 (2003).
Mendez, E. P. et al. US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 49, 300–305 (2003).
Acknowledgements
The authors are grateful to R. Rosenfield and J. L. Nelson for discussions, and to S. Khosla and E. Atkinson for providing primary data on sex-steroid levels in adults. The authors are supported in part by National Institutes of Health (NIH) grants HD021244, HL070831 and HL085197 (C.O.), GM077959 (Y.G.) and T32 HL07605 (D.A.L.). Y.G. is also supported by the Sloan foundation.
Author information
Authors and Affiliations
Corresponding author
Glossary
- Heterogametic
-
Refers to the sex that produces gametes that have two different sex chromosomes. In mammals, males are the heterogametic sex (XY) and females are homogametic (XX), whereas in birds females are heterogametic (ZW).
- Genetic architecture
-
Refers to the underlying genetic basis for a trait.
- Pyloric stenosis
-
A common birth defect that results from the narrowing of the pylorus (lower part of the stomach), which prevents food and other stomach contents from passing into the intestine. This condition causes severe vomiting in infancy. Also called infantile hypertrophic pyloric stenosis.
- Regulatory genome
-
The total set of different DNA molecules of an organelle, cell or organism that are involved in the regulation of gene expression.
- Sexual selection
-
Differential reproductive success resulting from the competition for fertilization, which can occur through competition among the same sex (mate competition) or through attraction to the opposite sex (mate choice).
- Ontogenetic conflict
-
Occurs when the same allele has different fitness consequences in juveniles and adults or in males and females.
- Expression QTL
-
(eQTL). Loci at which genetic allelic variation is associated with variation in gene expression.
- Alloimmune
-
An immune reaction against cells from another individual of the same species. Alloimmunity can occur during transfusion or transplantation, or during pregnancy.
- Heritability
-
The proportion of the total phenotypic variance for a given trait that can be attributed to genetic variation among individuals.
- Forced expiratory volume at 1 second
-
(FEV1). The volume exhaled in the first second of a forced expiratory manoeuvre. This index is used to assess airway obstruction, bronchoconstriction or bronchodilation.
- Type I error
-
The probability of rejecting the null hypothesis when it is true, also referred to as a false positive.
- Multiple testing
-
An analysis in which multiple independent hypotheses are tested. Multiple testing must be taken into account during statistical analysis, as the combined probability of type I error increases in an unadjusted analysis.
- Consomic strain
-
Inbred strain in which a chromosome has been replaced by a homologous chromosome from another inbred strain.
- Penetrance
-
The probability of observing a specific phenotype in individuals carrying a particular genotype.
- Linkage disequilibrium
-
(LD). The nonrandom association of alleles at two or more loci. The pattern of linkage disequilibrium in a given genomic region reflects the history of natural selection, mutation, recombination, genetic drift, and other demographic and evolutionary forces.
- Nondisjunction
-
The failure of chromosomes to separate at anaphase.
- Aneuploidy
-
The presence of an abnormal number of chromosomes, either more or less than the diploid number.
- Ectopic exchange
-
Homologous recombination between non-allelic chromosomal regions.
- Odds ratio
-
(OR). Compares the likelihood of an outcome (for example, a disease) between two groups (for example, cases and controls). It is measured as the ratio of the odds in one group to the odds in the second group and can be calculated by the following formula: OR = p(1 − q)/q(1 − p), where p is the probability of the event occurring for the first group and q the probability for the second group.
Rights and permissions
About this article
Cite this article
Ober, C., Loisel, D. & Gilad, Y. Sex-specific genetic architecture of human disease. Nat Rev Genet 9, 911–922 (2008). https://doi.org/10.1038/nrg2415
Issue Date:
DOI: https://doi.org/10.1038/nrg2415
This article is cited by
-
Preconceptional maternal hyperandrogenism and metabolic syndrome risk in male offspring: a long-term population-based study
Journal of Endocrinological Investigation (2024)
-
Phase field modelling and simulation of damage occurring in human vertebra after screws fixation procedure
Computational Mechanics (2024)
-
Sexual dimorphism in chronic respiratory diseases
Cell & Bioscience (2023)
-
Translational profiling identifies sex-specific metabolic and epigenetic reprogramming of cortical microglia/macrophages in APPPS1-21 mice with an antibiotic-perturbed-microbiome
Molecular Neurodegeneration (2023)
-
Evaluating adverse effects of environmental agents in food: a brief critique of the US FDA’s criteria
Environmental Health (2023)