Key Points
-
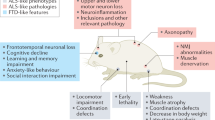
Genetically engineered mouse models have given us insights into the molecular pathogenesis of several neurological diseases. However, often they fail to accurately replicate all, or even most, of the features of the human disease. Nonetheless, careful analysis of the existing models points to the reasons for this failure.
-
It is necessary to accelerate the disease process to model the dominantly inherited neurodegenerative diseases within the normal lifespan of a mouse. Transgenic mice that overexpress the mutant proteins under potent heterologous promoters reproduce disease-like phenotypes; however, such models often display features that are a result of abnormal expression.
-
To generate accurate models for polyglutamine diseases, a knock-in strategy that targets long repeats that are predicted to cause infantile disease in humans is a valid approach as it eliminates the confounding effects of overexpression.
-
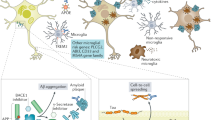
Reproducing the clinical and pathological features of diseases caused by missense and/or splicing mutations, such as α-synuclein-related Parkinson disease or tauopathy, is a challenge. The creation of a large collection of BAC- or YAC-based trangenics is worthy of consideration.
-
Generating faithful animal models for recessive diseases can not always be achieved through the conventional knockout approaches. Conditional gene disruption, or partial rescue of the knockout allele, might be necessary to model those diseases that are caused by insufficient levels or dysfunction of the protein.
-
The effects of skewed X-chromosome inactivation might complicate the phenotypic analysis of mouse models of X-linked disorders. Modelling an X-linked male-lethal disease can be also challenging since most ES cell lines are derived from the male germline.
-
Progress in manipulating the mouse genome, together with the development of new behavioural assays and imaging technologies, will markedly change the approaches to generating and analysing mouse models of brain diseases in the near future.
Abstract
Genetically engineered mice have been generated to model a variety of neurological disorders. Several of these models have provided valuable insights into the pathogenesis of the relevant diseases; however, they have rarely reproduced all, or even most, of the features observed in the corresponding human conditions. Here, we review the challenges that must be faced when attempting to accurately reproduce human brain disorders in mice, and discuss some of the ways to overcome them. Building on the knowledge gathered from the study of existing mutants, and on recent progress in phenotyping mutant mice, we anticipate better methods for preclinical interventional trials and significant advances in the understanding and treatment of neurological diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Otterbach, B. & Stoffel, W. Acid sphingomyelinase-deficient mice mimic the neurovisceral form of human lysosomal storage disease (Niemann-Pick disease). Cell 81, 1053–1061 (1995).
Horinouchi, K. et al. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nature Genet. 10, 288–293 (1995).
Pennachio, L. A. et al. Progressive ataxia, myoclonic epilepsy, and cerebellar apoptosis in cystatin B-deficient mice. Nature Genet. 20, 251–258 (1998).
Hardy, J. & Gwinn-Hardy, K. Genetic classification of primary neurodegenerative disease. Science 282, 1075–1079 (1998).
Taylor, J. P., Hardy, J. & Fischbeck, K. H. Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002).
Gusella, J. F. & MacDonald, M. E. Molecular genetics: unmasking polyglutamine triggers in neurodegnerative disease. Nature Rev. Neurosci. 1, 109–115 (2000).
Orr, H. T. & Zoghbi, H. Y. SCA1 molecular genetics: a history of a 13 year collaboration against glutamines. Hum. Mol. Genet. 10, 2307–2311 (2001).
Burright, E. et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 82, 937–948 (1995).
Matilla, A. et al. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J. Neurosci. 18, 5508–5516 (1998).
Fernandez-Funez, P. et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408, 101–106 (2000).
Klement, I. A. et al. Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95, 41–53 (1998).
Lorenzetti, D. et al. Repeat instability and motor incoordination in mice with a targeted expanded CAG repeat in the Sca1 locus. Hum. Mol. Genet. 9, 779–785 (2000).
Watase, K. et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron 34, 905–919 (2002). This paper reports on mice that express the mutant protein with endogenous patterns and replicate the selective neuronal vulnerability that is found in polyglutamine disorders.
Sobue, G. et al. Subclinical phenotypic expressions in heterozygous females of X–linked recessive bulbospinal neuronopathy. J. Neurol. Sci. 117, 74–78 (1993).
Ishihara, H. et al. Clinical features and skewed X-chromosome inactivation in female carriers of X-linked recessive spinal and bulbar muscular atrophy. J. Neurol. 248, 856–860 (2001).
Ferlini, A. et al. Androgen receptor (CAG)n repeat analysis in the differential diagnosis between Kennedy disease and other motor neuron disorders. Am. J. Med. Genet. 55, 105–111 (1995).
Zhou, Z. X., Wong, C. I., Sar, M. & Wilson, E. M. The androgen receptor: an overview. Recent Prog. Horm. Res. 49, 249–274 (1994).
Bingham, P. M. et al. Stability of an expanded trinucleotide repeat in the androgen receptor gene in transgenic mice. Nature Genet. 9, 191–196 (1995).
La Spada, A. R. et al. Androgen receptor YAC transgenic mice carrying CAG 45 alleles show trinucleotide repeat instability. Hum. Mol. Genet. 7, 959–967 (1998).
Abel, A. et al. Expression of expanded repeat androgen receptor produces neurologic disease in transgenic mice. Hum. Mol. Genet. 10, 107–116 (2001).
Ikeda, H. et al. Expanded polyglutamine in the Machado-Joseph disease protein induces cell death in vitro and in vivo. Nature Genet. 13, 196–202 (1996).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996).
McManamny, P. et al. A mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 18, 2103–2111 (2002).
Katsuno, M. et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002) Through the creation of a transgenic animal model expressing full-length mutant AR cDNA, this study indicated that nuclear translocation of the mutant AR by testosterone might contribute to the profound gender differences of phenotypes in SBMA and showed the therapeutic potential of hormonal intervention.
Yoo, S. Y. et al. SCA7 knock-in mice reproduce features of human SCA7 and reveal that mutant ataxin-7 gradually accumulates in neurons and interferes with short-term synaptic plasticity. Neuron 37, 383–401 (2003). By inserting highly expanded CAG repeat seen in juvenile cases of SCA7 into the corresponding mouse locus, the authors succeeded in generating an accurate model for this polyglutamine disease.
Mouradian, M. M. Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology 58, 179–185 (2002).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998)
Masliah, E. et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 (2000). This paper shows that the accumulation of wild-type α-synuclein causes dopaminergic neuron dysfunction and loss in transgenic mice.
van der Putten, H. et al. Neuropathology in mice expressing human α-synuclein. J. Neurosci. 20, 6021–6029 (2000).
Giasson, B. I. et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533 (2002).
Neumann, M. et al. Misfolded proteinase-K resistant hyperphosphorylated α-synuclein in aged transgenic mice with locomotor deterioration and in α-synucleinopathies. J. Clin. Invest. 110, 1429–1439 (2002).
Gomez-Isla, T. et al. Motor dysfunction and gliosis with preserved dopaminergic markers in human α-synuclein A30P transgenic mice. Neurobiol. Aging 24, 245–258 (2003).
Heintz, N. BAC to the future: the use of BAC transgenic mice for neuroscience research. Nature Rev. Neurosci. 2, 861–870 (2001). An excellent review of the usefulness of BAC transgenes as a tool for in vivo functional studies of genes implicated in nervous-system function and dysfunction.
Copeland, N. G., Jenkins, N. A. & Court, D. L. Recombineering: a powerful new tool for mouse functional genomics. Nature Rev. Genet. 2, 769–779 (2001) This review gives full details of the recent technological advances in a phage-based homologous recombination system that enables the modification and subcloning of genomic DNA in BACs.
Harding, A. E. Friedreich ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 104, 589–620 (1981)
Campuzano, V. et al. Friedreich ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 (1996)
Campuzano, V. et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum. Mol. Genet. 6, 1771–1780 (1997).
Rötig, A. et al. Frataxin gene expansion causes aconitase and mitochondrial iron-sulfer protein deficiency in Friedreich ataxia. Nature Genet. 17, 215–217 (1997).
Cossée, M. et al. Inactivation of Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9, 1219–1226 (2000).
Puccio, H. et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defects and Fe-S enzyme deficiency followed by mitochondrial iron deposits. Nature Genet. 27, 181–186 (2001). This paper reports on the creation of FRDA mouse models using conditional gene-targeting approaches. The mice reproduced both pathological and biochemical features of the human disease.
Miranda, C. J. et al. Frataxin knock-in mouse. FEBS Lett. 512, 291–297 (2002).
Pearn, J. Incidence, prevalence, and gene frequency studies of chronic spinal muscular atrophy. J. Med. Genet. 15, 409–413 (1978)
Lorson, C. L. & Androphy, E. J. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum. Mol. Genet. 9, 259–265 (2000)
Frugier, T., Nicole, S., Cifuentes-Diaz, C. & Melki, J. The molecular bases of spinal muscular atrophy. Curr. Opin. Genet. Dev. 12, 294–298 (2002)
Liu, Q. & Dreyfuss, G. A novel nuclear structure containing the survival motor neurons protein. EMBO J. 15, 3555–3564 (1996).
Schrank, B. et al. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl Acad. Sci. USA 96, 6307–6311 (1997)
Hsieh-Li, H. M. et al. A mouse model for spinal muscular atrophy. Nature Genet. 24, 66–70 (2000).
Monani, U. R. et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 9, 331–339 (2000). References 47 and 48 report the creation of transgenic mice carrying the entire human SMN2 gene on the Smn -null background. These approaches overcame the embryonic lethality seen in Smn -null mice, providing accurate mouse models for SMA and confirming that the disease severity is dependent on the copy number of SMN2 or levels of SMN protein.
Mastaglia, F. L. & Walton, J. N. Histological and histochemical changes from cases of chronic juvenile and early adult muscular spinal atrophy (the Kugelberg-Welander syndrome). J. Neurol. Sci. 12, 15–44 (1971).
Frugier, T. et al. Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscluar atrophy. Hum. Mol. Genet. 9, 849–858 (2000).
Cifuentes-Diaz, C. et al. Deletion of murine Smn exon 7 directed to skeletal muscle leads to severe muscular atrophy. J. Cell. Biol. 152, 1107–1114 (2001).
Chang, J. -G. et al. Treatment of spinal muscular atrophy by sodium butyrate. Proc. Natl Acad. Sci. USA 98, 9808–9813 (2001). This paper shows that sodium butyrate treatment of SMA-like mice resulted in the increased expression of SMN protein in motor neurons and improvement of the phenotypes, indicating that sodium butyrate might be an effective drug for treating SMA patients.
Lindsay, E. A. et al. Microphthalmia with linear skin defects (MLS) syndrome: clinical, cytogenetic, and molecular characterization. Am. J. Med. Genet. 49, 229–234 (1994).
Landy, S. J. & Donnai, D. Incontinentia pigmenti (Bloch-Sulzberger syndrome). J. Med. Genet. 30, 53–59 (1993).
Prakash, S. K. et al. Loss of holocytochrome c-type synthetase causes the male lethality of X-linked dominant microphtalmia with linear skin defects (MLS) syndrome. Hum. Mol. Genet. 11, 3237–3248 (2002).
Makris, C. et al. Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell 5, 969–979 (2000).
O'Donnel, W. T. & Warren, S. T. A decade of molecular studies of fragile X syndrome. Annu. Rev. Neurosci. 25, 315–338 (2002).
Dutch–Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78, 23–33 (1994).
Peier, A. M. et al. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum. Mol. Genet. 9, 1145–1159 (2000). By analysing YAC transgenic mice that overexpress FMR1 protein on a Fmr1 -null background, the authors suggested that the levels of FMR1 protein must be regulated to enable gene therapy to be considered for fragile X syndrome.
Hinton, V. J. Brown, W. T., Wisniewski, K. & Rudelli, R. D. Analysis of neocortex in three males with the fragile X syndrome. Am. J. Med. Genet. 41, 289–294 (1991).
Rudelli, R. D. et al. Adult fragile X syndrome: clinico-neuropathologic findings. Acta. Neuropathol. 67, 289–295 (1985).
Comery et al. Abnormal dendritic spines in fragile X knockouts mice: maturation and pruning deficits. Proc. Natl Acad. Sci. USA 94, 5401–5404 (1997).
Nimchinsky, E. A., Oberlander, A. M. & Svoboda, K. Abnormal development of dendritic spine in FMR1 knock-out mice. J. Neurosci. 21, 5139–5146 (2001).
Wan, M. et al. Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am. J. Hum. Genet. 65, 1520–1529 (1999).
Chen, R. Z. et al. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genet. 27, 327–331 (2001)
Guy, J. et al. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genet. 27, 322–326 (2001) References 65 and 66 indicate that total Mecp2 deficiency in male mice models the more severe neonatal encephalopathy seen in humans. Importantly, these papers show that the phenotype is a result of the loss of Mecp2 function in neurons.
Shahbazian, M. D. et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35, 243–254 (2002) The authors generate a hypomorphic allele of Mecp2 to avoid lethality and establish a Rett phenotype in male mice, avoiding the confounding effect of XCI.
Nicholls, R. D., Saitoh, S. & Horsthemke, B. Imprinting in Prader-Willi and Angelman syndrome. Trends Genet. 14, 194–200 (1998)
Williams, C. A. et al. Angelman syndrome. Curr. Prob. Pediatr. 25, 216–231 (1995)
Jiang, Y. et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21, 799–811 (1998)
Miura, K. et al. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol. Dis. 9, 149–159 (2002).
Reiner, O. et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature 364, 717–721 (1993).
Hirotsune, S. et al. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nature Genet. 19, 333–339 (1998). This work clearly indicates that Lis1 controls neuronal migration throughout the mouse brain in a dosage-dependent manner, by taking advantage of both null and hypomorphic alleles of the gene.
Biervert, C. et al. A potassium channel mutation in neonatal human epilepsy. Science 279, 403–436 (1998).
Singh, N. A. et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nature Genet. 18, 25–29 (1998)
Chalier, C. et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nature Genet. 18, 53–55 (1998).
Steinlein, O. K. et al. A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nature Genet. 11, 201–203 (1995).
De Fusco, M. et al. The nicotinic receptor β2 subunit is mutant in nocturnal frontal lobe epilepsy. Nature Genet. 26, 275–276 (2000).
Schroeder, B. C., Kubisch, C., Stein, V. & Jentsch, T. J. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature 396, 687–690 (1998).
Kuryatov A., Gerzanich V., Nelson, M., Olale, F. & Lindstrom, J. Mutation causing autosomal dominant frontal lobe epilepsy alters Ca2+ permeability, conductance, and gating of human α4β2 nicotinic acetylcholine receptors. J. Neurosci. 17, 9035–9047 (1997).
Phillips, H. A. et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am. J. Hum. Genet. 68, 225–231 (2001).
Cordero-Erauquin, M. et al. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol. Sci. 21, 211–217 (2000).
Baulac, S. et al. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nature Genet. 28, 46–48 (2001).
Wallace, R. H. et al. Mutant GABAA receptor γ–subunit in childhood absence epilepsy and febrile seizures. Nature Genet. 28, 49–52 (2001).
Günther, U. et al. Benzodiazepine-insensitive mice generated by targeted disruption of the γ2 subunit of γ-aminobutyric acid type A receptors. Proc. Natl Acad. Sci. USA 92, 7795–7753 (1995)
Burgess, D. L. & Noebels, J. Voltage-dependent calcium channel mutations in neurological disease. Ann. NY Acad. Sci. 868, 199–212 (1999).
Jun, K. et al. Ablation of P/Q type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking α1A subunit. Proc. Natl Acad. Sci. USA 96, 15245–15250 (1999).
Jouvenceau, A. et al. Human epilepsy associated with dysfunction of the brain P/Q type calcium channel. Lancet 358, 801–807 (2001).
Escayg, A. et al. Coding and noncoding variation of the human calcium-channel β4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am. J. Hum. Genet. 66, 1531–1539 (2000).
Chelly, J. & Mandel, J. -L. Monogenic causes of X-linked mental retardation. Nature Rev. Genet. 2, 669–680 (2001).
D'Adamo, P. et al. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum. Mol. Genet. 11, 2567–2580 (2002).
Gu, Y. et al. Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knock-out mice. J. Neurosci. 22, 2753–2763 (2002).
Jinnah, H. A. & Friedmann, T. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R., Beaudet, A.L., Sly, W. S. & Valle, D.) 2537–2570 (McGraw-Hill, New York, 2001).
Hooper, M., Hardy, K., Handyside, A., Hunter, S. & Monk, M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature 326, 292–295 (1987)
Kuehn, M. R., Bradley, A., Robertson, E. J. & Evans, M. J. A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations in mice. Nature 326, 295–298 (1987)
Mathis, C., Paul, S. N. & Crawley, J. N. Characterization of benzodiazepine-sensitive behaviors in the A/J and C57BL/6J inbred strains of mice. Behav. Genet. 2, 171–180 (1994)
Gerlai, R. Gene-targeting studies of the mammalian behavior: is it the mutation or the background phenotype? Trends Neurosci. 19, 177–181 (1996).
Gerlai, R. Contextual learning and cue association in fear conditioning in mice: a strain comparison and lesion study. Behav. Brain Res. 95, 191–203 (1998)
Taketo, M. et al. FVB/N: an inbred mouse strain preferable for transgenic analysis. Proc. Natl Acad. Sci. USA 88, 2065–2069 (1991).
Mineur, Y. S. & Crusio, W. E. Behavioral and neuroanatomical characterization of FVB/N inbred mice. Brain Res. Bull. 57, 41–47 (2002).
Carlson, G. A. et al. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum. Mol. Genet. 6, 1951–1959 (1997).
Kunst, C. B., Messer, L., Gordon, J., Haines, J. & Patterson, D. Genetic mapping of a mouse modifier gene that can prevent ALS onset. Genomics 70, 181–189 (2000).
Reaume, A. G. et al. Motor neurons in Cu/Zn superoxide dismutase deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genet. 13, 43–47 (1996).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Wong, P. C. et al. An adverse property of familial ALS linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Ripps, M. E., Huntley, G. W., Hof, P. R., Morrison, J. H. & Gordon, J. W. Transgenic mice expressing superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 92, 689–693 (1995).
Bruijn, L. I. et al. ALS linked SOD1 mutant G85R mediates damage to astrocyte and promotes rapidly progressive disease with SOD1 containing inclusions. Neuron 18, 327–338 (1997).
Muchowski, P. J. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 35, 9–12 (2002).
Cohen, F. E. Prions, peptides and protein misfolding. Mol. Med. Today 6, 292–293 (2000).
Davies, S. W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997)
Oshima, T. et al. α-galactosidase A deficient mice: a model of Fabry disease. Proc. Natl Acad. Sci. USA 94, 2540–2544 (1997).
Forss-Petter, S. et al. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J. Neurosci. Res. 50, 829–843 (1997).
Siatskas, C. & Medin, J. A. Gene therapy for Fabry disease. J. Inherit. Metab. Dis. 24 (Suppl.) 25–41 (2001).
Kemp, S. et al. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nature Med. 4, 1261–1268 (1998).
Netik, A. et al. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum. Mol. Genet. 8, 907–913 (1999).
van der Weyden, L., Adams, D. J. & Bradley, A. Tools for targeted manipulation of the mouse genome. Physiol. Genomics 11, 133–164 (2002). This review covers recent technological advances in mouse genomic engineering.
Lewandoski, M. Conditional control of gene expression in mouse. Nature Rev. Genet. 2, 743–755 (2001).
Hoehn, M. et al. Application of magnetic resonance to animal models of cerebral ischemia. J. Magn. Reson. Imaging 14, 491–509 (2001).
Koistinaho, M. et al. β-amyloid precursor transgenic mice that harbor diffuse Aβ deposits but do not form plaques show increased ischemic vulnerability: role of inflammation. Proc. Natl Acad. Sci. USA 99, 1610–1615 (2002).
Mueggler, T. et al. Compromised hemodynamic response in amyloid precursor protein transgenic mice. J. Neurosci. 22, 7218–7224 (2002).
Small, S. A. et al. Imaging physiologic dysfunction of individual hippocampal subregions in humans and genetically modified mice. Neuron 28, 653–664 (2000).
Skovronsky, D. M. et al. In vivo detection of amyloid plaques in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 97, 7609–7614 (2000).
Wengenack, T. M., Curran, G. L. & Podulso, J. F. Targeting Alzheimer amyloid plaques in vivo. Nature Biotechnol. 18, 868–872 (2000).
Bacskai, B. J. et al. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nature Med. 7, 369–372 (2001).
Poduslo, J. F. et al. Molecular targeting of Alzheimer's amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiol. Dis. 11, 315–329 (2002).
Ferrante, R. J. et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J. Neurosci. 20, 4389–4397 (2000).
Andreassen, O. A. et al. Creatine increases survival and delays motor symptoms in a transgenic animal model of Huntington's disease (2001). Neurobiol. Dis. 8, 479–491 (2001).
Ferrante, R. J. et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J. Neurosci. 22 1592–1599 (2002).
Ona, V. O. et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399, 263–267 (1999).
Andreassen O. A. et al. Dichloroacetate exerts therapeutic effects in transgenic mouse models of Huntington's disease. Ann. Neurol. 50, 112–116 (2001).
Dedeoglu, A. et al. Therapeutic effects of cystamine in a murine model of Huntington's disease. J. Neurosci. 22, 8942–8950 (2002).
Chen M. et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med. 6, 797–801 (2000).
Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology 57, 397–404 (2001).
Crawley, J. N. & Paylor, R. E. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 31, 197–211 (1997).
Hunter, A. J. Nolan, P. M. & Brown, S. D. M. Towards new models of disease and physiology in the neurosciences: the role of induced and naturally occurring mutations. Hum. Mol. Genet. 9, 893–900 (2000).
Bolivar, V., Cook, M. & Flaherty, L. List of transgenic and knockout mice: behavioral profiles. Mamm. Genome 11, 260–274 (2000)
Golub, M. S. & Germann, S. L. Long-term consequences of developmental exposure to aluminium in a suboptimal diet for growth and behavior of Swiss Webster mice. Neurotoxicol. Teratol. 23, 365–372 (2001).
Clark, H. B. et al. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J. Neurosci. 17, 7385–7395 (1997).
Giovannini M. et al. Conditional bialleic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes. Dev. 14, 1617–1630 (2000).
Kalamarides M. et al. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes. Dev. 16, 1060–1065 (2002).
Sango, K. et al. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nature Genet. 11, 170–176 (1995)
Phaneuf, D. et al. Dramatically different phenotypes in mouse models of human Tay-Sachs and Sandhoff diseases. Hum. Mol. Genet. 5, 1–14 (1996).
Yokota, T. et al. Delayed-onset ataxia in mice lacking α-tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl Acad. Sci. USA 98, 15185–15190 (2001).
Gencic, S. & Hudson, L. D. Conservative amino-acid substitution in the myelin proteolipid protein of jimpymsd mice. J. Neurosci. 10, 117–124 (1990).
Kagawa, T. et al. Glial cell degeneration and hypomyelination caused by overexpression of myelin proteolipid protein gene. Neuron 13, 427–442 (1994).
Klugmann, M. et al. Assembly of CNS myelin in the absence of proteolipid protein. Neuron 18, 59–70 (1997).
Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 (1998).
Lin, C. H. et al. Neurological abnormalities in a knock-in mouse model of Huntington's disease. Hum. Mol. Genet. 15, 137–144, (2001).
Yeh, S. et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc. Natl Acad. Sci. USA 99, 13498–13503 (2002).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
Databases
LocusLink
OMIM
dentatorubral pallidoluysian atrophy
microphthalmia with linear skin defects
progressive myoclonus epilepsy
Further information
Glossary
- LEWY BODIES
-
Intracytoplasmic enosinophilic round-to-elongated inclusions that are found in injured neurons. The Lewy body is a histological marker in Parkinson disease.
- HETEROLOGOUS PROMOTER
-
A DNA sequence that drives the transcription of a non-endogenous target gene that is placed in its adjacent region.
- CEREBELLUM
-
The part of the vertebrate hindbrain that modulates the force and range of movements, maintains balance and is involved in motor learning.
- BRAIN STEM
-
The portion of the brain between the cerebrum and the spinal cord, comprising the mesencephalon, pons and medulla.
- PURKINJE CELLS
-
The cerebellar neurons that convey the output signals of the cerebellar cortex.
- ACONITASES
-
The enzymes that catalyse the reversible hydration of cis-aconitase to yield citrate or isocitrate. They are involved in the citric acid cycle.
- DENERVATION
-
The removal of the nerve supply to a tissue.
- CRE-LOXP RECOMBINATION SYSTEM
-
A tool for tissue-specific knockout. It uses Cre recombinase from bacteriophage P1, which mediates intra- and intermolecular site-specific recombination between loxP sites.
- DYSTROPHIC
-
Cells or tissues that are undergoing progressive changes that might result from denervation or defective energy supply.
- DYSMORPHIC
-
A body characteristic that is abnormal as the result of a developmental defect.
- MACROORCHIDISM
-
The state of having abnormally large testes.
- ENCEPHALOPATHY
-
Altered cerebral function as a result of diffuse pathology.
- NESTIN-CRE TRANSGENE
-
A type of transgene, in which Cre recombinase is expressed in a neuronal lineage under the control of the nestin promoter.
- HYPOMORPHIC ALLELE
-
An allele that expresses a gene product with reduced activity.
- GYRI
-
The crests of convolutions in the cerebral cortex.
- CORTICAL LAYERING
-
The formation of six histologically identifiable layers in the cerebral cortex.
- NEURONAL HETEROTOPIAS
-
The displacement of neurons.
- PGK-NEO CASSETTE
-
A gene cassette that contains a neomycin-resistant gene under the control of the phosphoglycerol kinase gene promoter.
- ANTINOCICEPTIVE
-
Reduces the sensitivity to painful stimuli.
- FEBRILE SEIZURE PLUS
-
A syndrome in which patients have febrile seizure, plus an additional seizure subtype in adulthood.
- CHANNELOPATHIES
-
Diseases that are caused by defective ion-channel proteins.
- CEREBRAL ISCHAEMIA
-
A deficiency in the blood supply to the brain.
- HAEMODYNAMIC RESPONSES
-
The changes in blood flow, blood volume and blood oxygenation that occur in response to local neural activity.
Rights and permissions
About this article
Cite this article
Watase, K., Zoghbi, H. Modelling brain diseases in mice: the challenges of design and analysis. Nat Rev Genet 4, 296–307 (2003). https://doi.org/10.1038/nrg1045
Issue Date:
DOI: https://doi.org/10.1038/nrg1045
This article is cited by
-
Modeling human neurodevelopmental diseases with brain organoids
Cell Regeneration (2022)
-
Acamprosate in a mouse model of fragile X syndrome: modulation of spontaneous cortical activity, ERK1/2 activation, locomotor behavior, and anxiety
Journal of Neurodevelopmental Disorders (2017)
-
A glimmer of light for neuropsychiatric disorders
Nature (2008)
-
Technology Insight: querying the genome with microarrays—progress and hope for neurological disease
Nature Clinical Practice Neurology (2006)
-
SILencing misbehaving proteins
Nature Genetics (2005)