Abstract
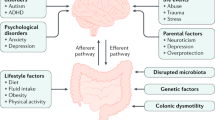
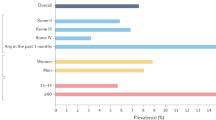
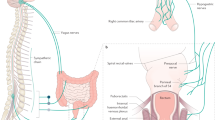
Chronic constipation is a prevalent condition that severely impacts the quality of life of those affected. Several types of primary chronic constipation, which show substantial overlap, have been described, including normal-transit constipation, rectal evacuation disorders and slow-transit constipation. Diagnosis of primary chronic constipation involves a multistep process initiated by the exclusion of ‘alarm’ features (for example, unintentional weight loss or rectal bleeding) that might indicate organic diseases (such as polyps or tumours) and a therapeutic trial with first-line treatments such as dietary changes, lifestyle modifications and over-the-counter laxatives. If symptoms do not improve, investigations to diagnose rectal evacuation disorders and slow-transit constipation are performed, such as digital rectal examination, anorectal structure and function testing (including the balloon expulsion test, anorectal manometry or defecography) or colonic transit tests (such as the radiopaque marker test, wireless motility capsule test, scintigraphy or colonic manometry). The mainstays of treatment are diet and lifestyle interventions, pharmacological therapy and, rarely, surgery. This Primer provides an introduction to the epidemiology, pathophysiological mechanisms, diagnosis, management and quality of life associated with the commonly encountered clinical problem of chronic constipation in adults unrelated to opioid abuse.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Peery, A. F. et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 149, 1731–1741 (2015).
Camilleri, M., Lembo, A. & Katzka, D. A. Opioids in gastroenterology: treating adverse effects and creating therapeutic benefits. Clin. Gastroenterol. Hepatol. 15, 1338–1349 (2017).
Talley, N. J., Zinsmeister, A. R., Van Dyke, C. & Melton, L. Epidemiology of colonic symptoms and the IBS. Gastroenterology 101, 927–934 (1991).
Walker, E. A., Katon, W. J., Jemelka, R. P. & Roy-Byrne, P. P. Comorbidity of GI complaints, depression, and anxiety in the epidemiologic catchment area (ECA) study. Am. J. Med. 92 (Suppl. 1), 26S–30S (1992).
Heaton, K. W. & Cripps, H. A. Straining at stool and laxative taking in an English population. Dig. Dis. Sci. 38, 1004–1008 (1993). This paper describes the stool form scale that allows assessment of severity of constipation and responses to treatment in the clinical and research setting.
Agreus, L., Svardsudd, K., Nyren, O. & Tibblin, G. The epidemiology of abdominal symptoms: prevalence and demographic characteristics in a Swedish adult population. Scand. J. Gastroenterol. 29, 102–109 (1994).
Suares, N. C. & Ford, A. C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am. J. Gastroenterol. 106, 1582–1591 (2011).
Heidelbaugh, J. J., Stelwagon, M., Miller, S. A., Shea, E. P. & Chey, W. D. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am. J. Gastroenterol. 110, 580–587 (2015).
Nullens, S. et al. Regional colon transit in patients with dyssynergic defecation or slow transit in patients with constipation. Gut 61, 1132–1139 (2012).
Rao, S. S. C. & Patcharatrakul, T. Diagnosis and treatment of dyssynergic defecation. J. Neurogastroenterol. Motil. 22, 423–435 (2016). This paper gives an update of the current management of defecation disorders.
Talley, N. J., Weaver, A. L., Zinsmeister, A. R. & Melton, L. J. 3rd . Functional constipation and outlet delay: a population-based study. Gastroenterology 105, 781–790 (1993).
Talley, N. J., Jones, M., Nuyts, G. & Dubois, D. Risk factors for chronic constipation based on a general practice sample. Am. J. Gastroenterol. 98, 1107–1111 (2003).
Papatheoridis, G. V., Vlachogiannakos, J., Karaitianos, I. & Karamanolis, D. G. A. Greek survey of community prevalence and characteristics of constipation. Eur. J. Gastroenterol. Hepatol. 22, 354–360 (2010).
Wald, A. et al. Survey of laxative use by adults with self-defined constipation in South America and Asia: a comparison of six countries. Aliment. Pharmacol. Ther. 31, 274–284 (2010).
Wald, A. et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment. Pharmacol. Ther. 28, 917–930 (2008).
Lovell, R. M. & Ford, A. C. Effect of gender on prevalence of IBS in the community: systematic review and meta-analysis. Am. J. Gastroenterol. 107, 991–1000 (2012).
Ford, A. C., Marwaha, A., Sood, R. & Moayyedi, P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut 64, 1049–1057 (2015).
McCrea, G. L. et al. Gender differences in self-reported constipation characteristics, symptoms, and bowel and dietary habits among patients attending a specialty clinic for constipation. Gender Med. 6, 259–271 (2009).
Lu, P. L., Velasco-Benítez, C. A. & Saps, M. Sex, age, and prevalence of pediatric irritable bowel syndrome and constipation in Colombia: a population-based study. J. Pediatr. Gastroenterol. Nutr. 64, e137–e141 (2017).
Inan, M. et al. Factors associated with childhood constipation. J. Paediatr. Child. Health. 43, 700–706 (2007).
Werth, B. L., Williams, K. A. & Pont, L. G. A longitudinal study of constipation and laxative use in a community-dwelling elderly population. Arch. Gerontol. Geriatr. 60, 418–424 (2015).
Schmidt, F. M., de Gouveia Santos, V. L., de Cássia Domansky, R. & Neves, J. M. Constipation: prevalence and associated factors in adults living in Londrina, Southern Brazil. Gastroenterol. Nurs. 39, 204–211 (2016).
Everhart, J. E. et al. A longitudinal survey of self-reported bowel habits in the United States. Dig. Dis. Sci. 34, 1153–1162 (1989).
Howell, S. C., Quine, S. & Talley, N. J. Low social class is linked to upper GI symptoms in an Australian sample of urban adults. Scand. J. Gastroenterol. 41, 657–666 (2006).
Bytzer, P. et al. Low socioeconomic class is a risk factor for upper and lower GI symptoms: a population based study in 15 000 Australian adults. Gut 49, 66–72 (2001).
Enck, P., Leinert, J., Smid, M., Kohler, T. & Schwille-Kiuntke, J. Prevalence of constipation in the German population — a representative survey (GECCO). United Eur. Gastroenterol. J. 4, 429–437 (2016).
Ebling, B. et al. Demographic, anthropometric and socioeconomic characteristics of functional constipation in Eastern Croatia. Coll. Antropol. 38, 539–546 (2014).
Nellesen, D. et al. Comorbidities in patients with IBS with constipation or chronic idiopathic constipation: a review of the literature from the past decade. Postgrad. Med. 125, 40–50 (2013).
Zhao, Y. F. et al. Epidemiology of functional constipation and comparison with constipation-predominant IBS: the Systematic Investigation of GI Diseases in China (SILC). Aliment. Pharmacol. Ther. 34, 1020–1029 (2011).
Zimmerman, J. & Hershcovici, T. Bowel symptoms in nonerosive gastroesophageal reflux disease: nature, prevalence, and relation to acid reflux. J. Clin. Gastroenterol. 42, 261–265 (2008).
Wong, R. K. et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am. J. Gastroenterol. 105, 2228–2234 (2010).
Mason, H. J., Serrano-Ikkos, E. & Kamm, M. A. Psychological morbidity in women with idiopathic constipation. Am. J. Gastroenterol. 95, 2852–2857 (2000).
Power, A. M., Talley, N. J. & Ford, A. C. Association between constipation and colorectal cancer: systematic review and meta-analysis of observational studies. Am. J. Gastroenterol. 108, 894–903 (2013).
Bharucha, A. E., Pemberton, J. H. & Locke, G. R. AGA technical review on constipation. Gastroenterology 144, 218–238 (2013).
Bayliss, W. M. & Starling, E. H. The movements and innervation of the small intestine. J. Physiol. 24, 99–143 (1899).
Nozdrachev, A. D. John Newport Langley and his autonomic (vegetative) nervous system structure (to the 150th anniversary of birth) [Russian]. Zh. Evol. Biokhim. Fiziol. 38, 422–429 (2002).
Reigstad, C. S. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403 (2015).
Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut — functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013).
Alemi, F. et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144, 145–154 (2013).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198.e16 (2017).
Wang, F. et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 595, 79–91 (2017).
Wattchow, D. A., Brookes, S. J. & Costa, M. The morphology and projections of retrogradely labeled myenteric neurons in the human intestine. Gastroenterology 109, 866–875 (1995).
Sanders, K. M., Ward, S. M. & Koh, S. D. Interstitial cells: regulators of smooth muscle function. Physiol. Rev. 94, 859–907 (2014).
McClain, J. L. et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146, 497–507 (2014).
Furness, J. B. et al. The enteric nervous system and GI innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71 (2014).
LePard, K. J., Ren, J. & Galligan, J. J. Presynaptic modulation of cholinergic and non-cholinergic fast synaptic transmission in the myenteric plexus of guinea pig ileum. Neurogastroenterol. Motil. 16, 355–364 (2004).
Szurszewski, J. H., Ermilov, L. G. & Miller, S. M. Prevertebral ganglia and intestinofugal afferent neurones. Gut 51 (Suppl. 1), i6–i10 (2002).
Berthoud, H. R., Carlson, N. R. & Powley, T. L. Topography of efferent vagal innervation of the rat GI tract. Am. J. Physiol. 260, R200–R207 (1991).
De Groat, W. C. & Krier, J. The sacral parasympathetic reflex pathway regulating colonic motility and defecation in the cat. J. Physiol. 276, 481–500 (1978).
Holzknechtg, G. Die normale persistatlik des kolon [German]. Muench. Med. Wochenschr. 47, 2401–2403 (1909).
Alvarez, W. C. in An Introduction to Gastroenterology 4th edn (ed. Alvarez, W. C. ) 325–363 (William Heinemann Medical Books Ltd., 1948).
Narducci, F., Bassotti, G., Gaburri, M. & Morelli, A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut 28, 17–25 (1987).
Bassotti, G. & Gaburri, M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am. J. Physiol. 255, G660–G664 (1988).
Rao, S. S., Sadeghi, P., Beaty, J., Kavlock, R. & Ackerson, K. Ambulatory 24-h colonic manometry in healthy humans. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G629–G639 (2001).
Furukawa, Y. et al. Relationship between sleep patterns and human colonic motor patterns. Gastroenterology 107, 1372–1381 (1994).
Bampton, P. A., Dinning, P. G., Kennedy, M. L., Lubowski, D. Z. & Cook, I. J. Prolonged multi-point recording of colonic manometry in the unprepared human colon: providing insight into potentially relevant pressure wave parameters. Am. J. Gastroenterol. 96, 1838–1848 (2001).
Torsoli, A. Ramorino, M. L., Ammaturo, M. V., Capurso, L., Paoluzi, P. & Anzini, F. Mass movements and intracolonic pressures. Am. J. Dig. Dis. 16, 693–696 (1971).
Hardcastle, J. D. & Mann, C. V. Study of large bowel peristalsis. Gut 9, 512–520 (1968).
Kamm, M. A., van der Sijp, J. R. & Lennard-Jones, J. E. Observations on the characteristics of stimulated defecation in severe idiopathic constipation. Int. J. Colorectal Dis. 7, 197–201 (1992).
Bampton, P. A., Dinning, P. G., Kennedy, M. L., Lubowski, D. Z. & Cook, I. J. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G443–G449 (2002).
De Schryver, A. M., Samsom, M. & Smout, A. I. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Dig. Dis. Sci. 48, 1206–1212 (2003).
Jouet, P. et al. Fermentation of starch stimulates propagated contractions in the human colon. Neurogastroenterol. Motil. 23, 450–e176 (2011).
Cook, I. J., Furukawa, Y., Panagopoulos, V., Collins, P. J. & Dent, J. Relationships between spatial patterns of colonic pressure and individual movements of content. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G329–G341 (2000).
Bampton, P. A. et al. Spatial and temporal organization of pressure patterns throughout the unprepared colon during spontaneous defecation. Am. J. Gastroenterol. 95, 1027–1035 (2000).
Hertz, A. F. Lectures on the passage of food through the human alimentary canal: delivered at Guy's Hospital for London University Advanced Students during October, 1907. Br. Med. J; 1, 130–137 (1908).
Lubowski, D. Z., Meagher, A. P., Smart, R. C. & Butler, S. P. Scintigraphic assessment of colonic function during defecation. Int. J. Colorect. Dis. 10, 91–93 (1995).
Ritchie, J. A. Colonic motor activity and bowel function. I. Normal movement of contents. Gut 9, 442–456 (1968).
Halls, J. Bowel content shift during normal defecation [summary]. Proc. R. Soc. Med. 58, 859–860 (1965).
Hiroz, P., Schlageter, V., Givel, J. C. & Kucera, P. Colonic movements in healthy subjects as monitored by a Magnet Tracking System. Neurogastroenterol. Motil. 21, e838–e857 (2009).
Moreno-Osset, E. et al. Association between postprandial changes in colonic intraluminal pressure and transit. Gastroenterology 96, 1265–1273 (1989).
Dinning, P. G., Szczesniak, M. M. & Cook, I. J. Proximal colonic propagating pressure waves sequences and their relationship with movements of content in the proximal human colon. Neurogastroenterol. Motil. 20, 512–520 (2008).
Dinning, P. G. et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol. Motil. 26, 1443–1457 (2014).
Lin, A. Y. et al. High-resolution anatomic correlation of cyclic motor patterns in the human colon: Evidence of a rectosigmoid brake. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G508–G515 (2017).
Rao, S. S. C., Sadeghi, P. & Beaty, J. Altered Periodic Rectal Motor Activity (PRMA): a mechanism for slow transit constipation. Neurogastroenterol. Motil. 13, 591–598 (2002).
Kern, F. Jr., Almy, T. P., Abbot, F. K. & Bogdonoff, M. D. The motility of the distal colon in nonspecific ulcerative colitis. Gastroenterology 19, 492–503 (1951).
Bazzocchi, G. et al. Effect of eating on colonic motility and transit in patients with functional diarrhea: simulataneous scintigraphic and manometric evaluation. Gastroenterology 101, 1298–1306 (1991).
von der Ohe, M. R., Hanson, R. B. & Camilleri, M. Comparison of simultaneous recordings of human colonic contractions by manometry and a barostat. Neurogastroenterol. Motil. 6, 213–222 (1994).
Schey, R., Cromwell, J. & Rao, S. Medical and surgical management of pelvic floor disorders affecting defecation. Am. J. Gastroenterol. 107, 1624–1633 (2012).
Rao, S. S. C., Welcher, K. D. & Leistikow, J. S. Obstructive defecation: a failure of rectoanal coordination. Am. J. Gastroenterol. 93, 1042–1050 (1998).
Rao, S. S. C., Mudipalli, R. S., Stessman, M. & Zimmerman, B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (anismus). Neurogastroenterol. Motil. 16, 589–596 (2004).
Rao, S. S. C., Tuteja, A. K., Vellema, T., Kempf, J. & Stessman, M. Dyssynergic defecation: demographics, symptoms, stool patterns, and QOL. J. Clin. Gastroenterol. 38, 680–685 (2004).
Karasick, S. & Ehrlich, S. M. Is constipation a disorder of defecation or impaired motility? Distinction based on defecography and colonic transit studies. Am. J. Roentgenol. 166, 63 (1996).
Preston, D. M. & Lennard-Jones, E. Anismus in chronic constipation. Dig. Dis. Sci. 30, 413–418 (1985).
Rao, S. S. C., Kavlock, R. & Rao, S. Influence of body position and stool characteristics on defecation in humans. Am. J. Gastroenterol. 101, 2790–2796 (2006).
Inho, M., Yoshioka, K. & Keighley, M. R. B. Long term results of anorectal myectomy for chronic constipation. Br. J. Surg. 76, 1163–1164 (1989).
Ron, Y. et al. Botulinum toxin type-A in therapy of patients with anismus. Dis. Colon Rectum 44, 1821–1826 (2001).
Remes-Troche, M. et al. Anorectal cortical function is impaired in patients with dyssynergic defecation. Gastroenterology 108, A20 (2007).
Rao, S. S. C. et al. Does biofeedback therapy modulate anorectal (gut)-brain axis in patients with dyssynergic defecation? Gastroenterology 140, S367 (2011).
Stivland, T. et al. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology 101, 107–115 (1991).
McLean, R. G. et al. Colon transit scintigraphy using oral indium-111-labeled DTPA: can scan pattern predict final diagnosis? Dig. Dis. Sci. 40, 2660–2668 (1995).
Bassotti, G. et al. Colonic mass movements in idiopathic chronic constipation. Gut 29, 1173–1179 (1988).
Dinning, P. G. et al. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol. Motil. 22, e340–e349 (2010). This paper maps the colonic contractility and its derangement in patients with severe chronic constipation.
Bazzocchi, G. et al. Postprandial colonic transit and motor activity in chronic constipation. Gastroenterology 96, 686–693 (1990).
Knowles, C. H., Scott, S. M. & Lunniss, P. J. Slow transit constipation: a disorder of pelvic autonomic nerves? Dig. Dis. Sci. 46, 389–401 (2001).
Singal, A. K., Rosman, A. S., Bauman, W. A. & Korsten, M. A. Recent concepts in the management of bowel problems after spinal cord injury. Adv. Med. Sci. 51, 15–22 (2006).
Lee, J. I., Park, H., Kamm, M. A. & Talbot, I. C. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J. Gastroenterol. Hepatol. 20, 1292–1298 (2005).
Cohen, M. et al. Evaluation of interstitial cells of Cajal in patients with severe colonic inertia requiring surgery: a clinical-pathological study. Colorectal Dis. 19, 462–466 (2017).
Gattuso, J. M. & Kamm, M. A. Clinical features of idiopathic megarectum and idiopathic megacolon. Gut 41, 93–99 (1997).
O’Dwyer, R. H. et al. Clinical features and colonic motor disturbances in chronic megacolon in adults. Dig. Dis. Sci. 60, 2398–2407 (2015).
Gibbons, D., Camilleri, M., Nelson, A. D. & Eckert, D. Characteristics of chronic megacolon among patients diagnosed with multiple endocrine neoplasia type 2B. United Eur. Gastroenterol. J. 4, 449–454 (2016).
Barrett, K. E. Endogenous and exogenous control of gastrointestinal epithelial function: building on the legacy of Bayliss and Starling. J. Physiol. 595, 423–432 (2017).
Hammer, J. & Phillips, S. F. Fluid loading of the human colon: effects on segmental transit and stool composition. Gastroenterology 105, 988–998 (1993).
Camilleri, M. et al. Effect of a chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory and motor functions in healthy humans. Am. J. Physiol. 290, G942–G947 (2006).
Andresen, V. et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 133, 761–768 (2007).
Barrett, K. E. & Keely, S. J. in Physiology of the Gastrointestinal Tract 4th edn (eds Johnson, L. R., Barrett, K. E., Gishan, F. K., Merchant, J. L., Said, H. M. & Wood, J. D. ) 1931–1951 (Elsevier Academic Press, 2006).
Zachos, N. C., Tse, M. & Donowitz, M. Molecular physiology of intestinal Na+/H+ exchange. Annu. Rev. Physiol. 67, 441–443 (2005).
Alli, A. A. et al. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol. Renal Physiol. 309, F456–F463 (2015).
Kashlan, O. B. & Kleyman, T. R. Epithelial Na+ channel regulation by cytoplasmic and extracellular factors. Exp. Cell. Res. 318, 1011–1019 (2012).
Malsure, S. et al. Colon-specific deletion of epithelial sodium channel causes sodium loss and aldosterone resistance. J. Am. Soc. Nephrol. 25, 1453–1464 (2014).
Bergann, T. et al. Glucocorticoids and tumor necrosis factor-alpha synergize to induce absorption by the epithelial sodium channel in the colon. Gastroenterology 136, 933–942 (2009).
Sellin, J. H. & De Soignie, R. Ion transport in human colon in vitro. Gastroenterology 93, 441–448 (1987).
Lacy, B. E. et al. Bowel disorders. Gastroenterology 150, 1393–1407.e5 (2016).
Lewis, S. J. & Heaton, K. W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 32, 920–924 (1997).
Degen, L. P. & Phillips, S. F. How well does stool form reflect colonic transit? Gut 39, 109–113 (1996).
Saad, R. J. et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am. J. Gastroenterol. 105, 403–411 (2010).
Wald, A., Bharucha, A. E., Cosman, B. C. & Whitehead, W. E. ACG Clinical Guideline: Management of benign anorectal disorders. Am. J. Gastroenterol. 109, 1141–1157 (2014).
Tantiphlachiva, K., Rao, P., Attaluri, A. & Rao, S. S. C. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin. Gastroenterol. Hepatol. 8, 955–960 (2010).
Soh, J. S. et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am. J. Gastroenterol. 110, 1197–1204 (2015).
Wong, R. K. et al. The digital rectal examination: a multicenter survey of physicians’ and students’ perceptions and practice patterns. Am. J. Gastroenterol. 107, 1157–1163 (2012).
Voderholzer, W. A. et al. Clinical response to dietary fiber treatment of chronic constipation. Am. J. Gastroenterol. 92, 95–98 (1997).
Rao, S. S., Rattanakovit, K. & Patcharatrakul, T. Diagnosis and management of chronic constipation in adults. Nat. Rev. Gastroenterol. Hepatol. 13, 295–305 (2016).
Minguez, M. et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology 126, 57–62 (2004).
Chiarioni, G. et al. Validation of the balloon evacuation test: reproducibility and agreement with findings from anorectal manometry and electromyography. Clin. Gastroenterol. Hepatol. 12, 2049–2054 (2014).
Bharucha, A. E. & Rao, S. S. An update on anorectal disorders for gastroenterologists. Gastroenterology 146, 37–45 (2014).
Rao, S. S. C. et al. Characterization of dyssynergia phenotypes with high resolution anorectal manometry (HRAM). Gastroenterology 150, S158–S159 (2016).
Patcharatrakul, T., Shaffer, N., DeWitt, A., Mack, A. & Rao, S. S. C. Does the type of dyssynergia influence the outcome of biofeedback therapy? Neurogastroenterol. Motil. 28 (Suppl. 1), 18 (2016).
Ratuapli, S. K., Bharucha, A. E., Noelting, J., Harvey, D. M. & Zinsmeister, A. R. Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology 144, 314–322 (2013).
Patcharatrakul, T. et al. Barostat-assisted sensory training (BT) is superior to syringe-assisted training (ST) for rectal hyposensitivity. Neurogastroenterol. Motil. 28 (Suppl. 1), 42 (2016).
Grossi, U. et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut 65, 447–455 (2016).
Kim, A. Y. How to interpret a functional or motility test — defecography. J. Neurogastroenterol. Motil. 17, 416–420 (2011).
Flushing, M., Sahni, V. A., Erturk, S. M. & Mortelel, K. J. Dynamic MR defecography: assessment of the usefulness of defecation phase. Am. J. Roentgenol. 196, W394–W399 (2011).
Foti, P. V. et al. Pelvic floor imaging: comparison between MRI and conventional defecography in studying outlet obstruction syndrome. Radiol. Med. 118, 23–39 (2013).
Kim, E. R. & Rhee, P. L. How to interpret a functional or motility test — colon transit study. J. Neurogastroenterol. Motil. 18, 94–99 (2012).
Rao, S. S. et al. Evaluation of GI transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol. Motil. 23, 8–23 (2011).
Hinton, J. M., Lennard-Jones, J. E. & Young, A. C. A new method for studying gut transit times using radioopaque markers. Gut 10, 842–847 (1969).
Saad, R. J. The wireless motility capsule: a one-stop shop for the evaluation of GI motility disorders. Curr. Gastroenterol. Rep. 18, 14 (2016).
Camilleri, M. et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol. Motil. 22, 874–882 (2010).
Rao, S. S. et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin. Gastroenterol. Hepatol. 7, 537–544 (2009).
Kuo, B. et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or STC. Dig. Dis. Sci. 56, 2928–2938 (2011).
Bonapace, E. S. et al. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower GI symptoms. Am. J. Gastroenterol. 95, 2838–2847 (2000).
Burton, D. D., Camilleri, M., Mullan, B. P., Forstrom, L. A. & Hung, J. C. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J. Nucl. Med. 38, 1807–1810 (1997).
Maurer, A. H. & Parkman, H. P. Update on GI scintigraphy. Semin. Nucl. Med. 36, 110–118 (2006).
Parkman, H. P. Scintigraphy for evaluation of patients for GI motility disorders — the referring physicians perspective. Semin. Nucl. Med. 42, 76–78 (2012).
Vitton, V. et al. Water-perfused manometry versus 3D high resolution manometry: a comparative study on a large patient population with anorectal disorders. Colorectal Dis. 15, e726–e731 (2013).
Di Lorenzo, C., Flores, A. F., Reddy, S. N. & Hyman, P. E. Use of colonic manometry to differentiate causes of intractable constipation in children. J. Pediatr. 120, 690–695 (1992).
Singh, S., Heady, S., Coss-Adame, E. & Rao, S. S. C. Clinical utility of colonic manometry in slow transit constipation. Neurogastroenterol. Motil. 25, 487–e367 (2013).
Huang, L. et al. Prevalence and risk factors of chronic constipation among women aged 50 years and older in Shanghai. China. Med. Sci. Monit. 23, 2660–2667 (2017).
Markland, A. D. et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am. J. Gastroenterol. 108, 796–803 (2013).
Sandler, R. S., Jordan, M. C. & Shelton, B. J. Demographic and dietary determinants of constipation in the US population. Am. J. Publ. Health 80, 185–189 (1990).
Muller-Lissner, S. A., Kamm, M. A., Scarpignato, C. & Wald, A. Myths and misconceptions about chronic constipation. Am. J. Gastroenterol. 100, 232–242 (2005).
Ziegenhagen, D. J., Tewinkel, G., Kruis, W. & Herrmann, F. Adding more fluid to wheat bran has no significant effects on intestinal functions of healthy subjects. J. Clin. Gastroenterol. 13, 525–530 (1991).
De Giorgio, R. et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 15, 130 (2015).
Mearin, F. et al. Clinical Practice Guideline: IBS with constipation and functional constipation in the adult. Rev. Esp. Enferm. Dig. 108, 332–363 (2016).
Ford, A. C. et al. American College of Gastroenterology monograph on the management of IBS and chronic idiopathic constipation. Am. J. Gastroenterol. 109 (Suppl. 1), S2–S26 (2014).
Badiali, D. et al. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig. Dis. Sci. 40, 349–356 (1995).
Bijkerk, C. J., Muris, J. W., Knottnerus, J. A., Hoes, A. W. & de Wit, N. J. Systematic review: the role of different types of fibre in the treatment of IBS. Aliment. Pharmacol. Ther. 19, 245–251 (2004).
Rao, S. S., Yu, S. & Fedewa, A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and IBS. Aliment. Pharmacol. Ther. 41, 1256–1270 (2015).
Suares, N. C. & Ford, A. C. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment. Pharmacol. Ther. 33, 895–901 (2011).
Bijkerk, C. J. et al. Soluble or insoluble fibre in IBS in primary care? Randomised placebo controlled trial. BMJ 339, b3154 (2009).
Francis, C. Y. & Whorwell, P. J. Bran and IBS: time for reappraisal. Lancet 344, 39–40 (1994).
Miller, V., Lea, R., Agrawal, A. & Whorwell, P. J. Bran and IBS: the primary-care perspective. Dig. Liver Dis. 38, 737–740 (2006).
McRorie J. W. Jr & McKeown, N. M. Understanding the physics of functional fibers in the GI tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 117, 251–264 (2017).
Johannesson, E. Simren, M., Strid, H., Bajor, A. & Sadik, R. Physical activity improves symptoms in IBS: a randomized controlled trial. Am. J. Gastroenterol. 106, 915–922 (2011).
Karam, S. E. & Nies, D. M. Student/staff collaboration: a pilot bowel management program. J. Gerontol. Nurs. 20, 32–40 (1994).
Dipalma, J. A. et al. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am. J. Gastroenterol. 102, 1436–1441 (2007).
Attar, A. et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 44, 226–230 (1999).
Cinca, R., Chera, D., Gruss, H. J. & Halphen, M. Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation — a comparison in a controlled environment. Aliment. Pharmacol. Ther. 37, 876–886 (2013).
Bass, P. & Dennis, S. The laxative effects of lactulose in normal and constipated subjects. J. Clin. Gastroenterol. 3 (Suppl. 1), 23–28 (1981).
Dupont, C., Campagne, A. & Constant, F. Efficacy and safety of a magnesium sulfate-rich natural mineral water for patients with functional constipation. Clin. Gastroenterol. Hepatol. 12, 1280–1287 (2014).
Ikarashi, N. et al. The laxative effect of bisacodyl is attributable to decreased aquaporin-3 expression in the colon induced by increased PGE2 secretion from macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G887–G895 (2011).
Manabe, N., Cremonini, F., Camilleri, M., Sandborn, W. J. & Burton, D. D. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment. Pharmacol. Ther. 30, 930–936 (2009).
Kamm, M. A. et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin. Gastroenterol. Hepatol. 9, 577–583 (2011).
Mueller-Lissner, S. et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am. J. Gastroenterol. 105, 897–903 (2010).
Nelson, A. D. et al. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis. Gut 66, 1611–1622 (2017). This paper is the first-ever network meta-analysis of pharmacotherapies for chronic constipation.
Milner, P. et al. Effects of long-term laxative treatment on neuropeptides in rat mesenteric vessels and caecum. J. Pharm. Pharmacol. 44, 777–779 (1992).
Johanson, J. F., Morton, D., Geenen, J. & Ueno, R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am. J. Gastroenterol. 103, 170–177 (2008).
Chey, W. D. et al. Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with IBS with constipation. Aliment. Pharmacol. Ther. 35, 587–599 (2012).
Lembo, A. J. et al. Two randomized trials of linaclotide for chronic constipation. N. Engl. J. Med. 365, 527–536 (2011).
Lacy, B. E. et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: a randomized, controlled trial. PLoS ONE 10, e0134349 (2015).
Miner, P. B. et al. Randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am. J. Gastroenterol. 112, 613–621 (2017).
Shin, A. et al. Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment. Pharmacol. Ther. 39, 239–253 (2014).
Camilleri, M., Kerstens, R., Rykx, A. & Vandeplassche, L. A placebo-controlled trial of prucalopride for severe chronic constipation. N. Engl. J. Med. 358, 2344–2354 (2008).
Chiarioni, G., Whitehead, W. E., Pezza, V., Morelli, A. & Bassotti, G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 130, 657–664 (2006). This paper describes the first randomized controlled trial demonstrating the efficacy of biofeedback training for dyssynergic defecation.
Heymen, S. et al. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Dis. Colon Rectum 42, 1388–1393 (1999).
Rao, S. S. et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin. Gastroenterol. Hepatol. 5, 331–338 (2007).
Lee, H. J. et al. Long-term efficacy of biofeedback therapy in patients with dyssynergic defecation: results of a median 44 months follow-up. Neurogastroenterol. Motil. 27, 787–795 (2015).
Rao, S. S. C. et al. ANMS-ESMN position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol. Motil. 27, 594–609 (2015).
Redmond, J. M. et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am. J. Gastroenterol. 90, 748–753 (1995).
Paquette, I. M. et al. The American Society of Colon and Rectal Surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis. Colon Rectum 59, 479–492 (2016).
Thaha, M. A., Abukar, A. A., Thin, N. N., Ramsanahie, A. & Knowles, C. H. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst. Rev. 8, CD004464
Graf, W., Sonesson, A. C., Lindberg, B., Åkerud, P. & Karlbom, U. Results after sacral nerve stimulation for chronic constipation. Neurogastroenterol. Motil. 27, 734–739 (2015).
Dinning, P. G. et al. Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am. J. Gastroenterol. 110, 733–740 (2015).
Patton, V., Stewart, P., Lubowski, D. Z., Cook, I. J. & Dinning, P. G. Sacral nerve stimulation fails to offer long-term benefit in patients with slow-transit constipation. Dis. Colon Rectum 59, 878–885 (2016).
Maeda, Y. et al. Long-term outcome of sacral neuromodulation for chronic refractory constipation. Tech. Coloproctol. 21, 277–286 (2017).
Zerbib, F. et al. Randomized clinical trial of sacral nerve stimulation for refractory constipation. Br. J. Surg. 104, 205–213 (2017).
He, C. L. et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterol. 118, 14–21 (2000).
Lyford, G. L. et al. Pan-colonic decrease in interstitial cells of Cajal in patients with STC. Gut 51, 496–501 (2002).
Pinedo, G. et al. Laparoscopic total colectomy for colonic inertia: surgical and functional results. Surg. Endosc. 23, 62–65 (2009).
Indar, A. A., Efron, J. E. & Young-Fadok, T. M. Laparoscopic ileal pouch-anal anastomosis reduces abdominal and pelvic adhesions. Surg. Endosc. 23, 174–177 (2009).
O’Brien, S., Hyman, N., Osler, T. & Rabinowitz, T. Sexual abuse: a strong predictor of outcomes after colectomy for slow-transit constipation. Dis. Colon Rectum 52, 1844–1847 (2009).
Nyam, D. C., Pemberton, J. H., Ilstrup, D. M. & Rath, D. M. Long-term results of surgery for chronic constipation. Dis. Colon Rectum 40, 273 (1997).
Knowles, C. H., Scott, M. & Lunniss, P. J. Outcome of colectomy for STC. Ann. Surg. 230, 627 (1999).
Hassan, I. et al. Ileorectal anastomosis for STC: long-term functional and QOL results. J. Gastrointest. Surg. 10, 1330–1336 (2006).
Pikarsky, A. J. et al. Long-term follow-up of patients undergoing colectomy for colonic inertia. Dis. Colon Rectum 44, 179 (2001).
FitzHarris, G. P. et al. Quality of life after subtotal colectomy for slow-transit constipation: both quality and quantity count. Dis. Colon Rectum 46, 433–440 (2003).
Belsey, J., Greenfield, S., Candy, D. & Geraint, M. Systematic review: impact of constipation on QOL in adults and children. Aliment. Pharmacol. Ther. 31, 938–949 (2010).
Wald, A. et al. The burden of constipation on QOL: results of a multinational survey. Aliment. Pharmacol. Ther. 26, 227–236 (2007).
Jiang, Y. et al. Influence of sleep disorders on somatic symptoms, mental health, and QOL in patients with chronic constipation. Medicine 96, e6093 (2017).
Glia, A. & Lindberg, G. QOL in patients with different types of functional constipation. Scand. J. Gastroenterol. 32, 1083–1089 (1997).
Marquis, P., De La Loge, C., Dubois, D., McDermott, A. & Chassany, O. Development and validation of the Patient Assessment of Constipation QOL questionnaire. Scand. J. Gastroenterol. 40, 540–551 (2005).
Bellini, M. et al. Chronic constipation diagnosis and treatment evaluation: the “CHRO.CO.DI.T.E.” study. BMC Gastroenterol. 17, 11 (2017).
Cai, Q. et al. Healthcare costs among patients with chronic constipation: a retrospective claims analysis in a commercially insured population. J. Med. Econ. 17, 148–158 (2014).
Nyrop, K. A. et al. Costs of health care for IBS, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment. Pharmacol. Ther. 26, 237–248 (2007).
Choung, R. S. et al. Longitudinal direct medical costs associated with constipation in women. Aliment. Pharmacol. Ther. 33, 251–260 (2011).
Mitra, D., Davis, K. L. & Baran, R. W. All-cause health care charges among managed care patients with constipation and comorbid IBS. Postgrad. Med. 123, 122–132 (2011).
Lembo, A. & Camilleri, M. Chronic constipation. N. Engl. J. Med. 349, 1360–1368 (2003). This review details the history and physical examination findings and mechanisms leading to rectal evacuation disorders among patients with chronic constipation.
Halder, S. L. et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 133, 799–807 (2007).
Kolar, G. J., Camilleri, M., Burton, D., Nadeau, A. & Zinsmeister, A. R. Prevalence of colonic motor or evacuation disorders in patients presenting with chronic nausea and vomiting evaluated by a single gastroenterologist in a tertiary referral practice. Neurogastroenterol. Motil. 26, 131–138 (2014).
Mohammed, S. D. et al. Joint hypermobility and rectal evacuatory dysfunction: an etiological link in abnormal connective tissue? Neurogastroenterol. Motil. 22, 1085–e1283 (2010).
Nelson, A. D. et al. Ehlers Danlos syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. Neurogastroenterol. Motil. 27, 1657–1666 (2015).
Fikree, A., Chelimsky, G., Collins, H., Kovacic, K. & Aziz, Q. Gastrointestinal involvement in the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 175, 181–187 (2017).
Wu, G. J., Xu, F., Lin, L., Pasricha, P. J. & Chen, J. D. Z. Anorectal manometry: should it be performed in a seated position? Neurogastroenterol. Motil. 29, e12997 (2017).
Clarke, M. C. et al. Transabdominal electrical stimulation increases colonic propagating pressure waves in paediatric slow transit constipation. J. Pediatr. Surg. 47, 2279–2284 (2012).
Gaertner, J. et al. Definitions and outcome measures of clinical trials regarding opioid-induced constipation: a systematic review. J. Clin. Gastroenterol. 49, 9–16 (2015).
Benninga, M. A. et al. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 150, 1443–1455.e2 (2016).
Hyams, J. S. et al. Functional disorders: children and adolescents. Gastroenterology 150, 1456–1468 (2016).
Tse, Y. et al. Treatment algorithm for chronic idiopathic constipation and constipation-predominant irritable bowel syndrome derived from a Canadian national survey and needs assessment on choices of therapeutic agents. Can. J. Gastroenterol. Hepatol. 2017, 8612189 (2017). This paper contains a recent guideline and algorithm for the management of chronic constipation.
Acknowledgements
This study was supported in part by the US Public Health Service NIH Grant 5R21DK104127-02 and Grant 1 U34 DK109191-01 to S.S.R. The authors thank C. L. Stanislav (Mayo Clinic, USA) for her excellent administrative support.
Author information
Authors and Affiliations
Contributions
Introduction (M.C.); Epidemiology (A.C.F.); Mechanisms/pathophysiology (M.C., G.M.M., P.G.D., S.S.R. and W.D.C.); Diagnosis, screening and prevention (W.D.C.); Management (M.S., A.L. and T.M.Y.-F.); Quality of life (L.C.); Outlook (M.C.); Overview of Primer (M.C.).
Corresponding author
Ethics declarations
Competing interests
M.C. serves on an advisory board for Ironwood Pharmaceuticals and Shire Pharmaceuticals, with compensation to his employer and not to him personally, and has received grant and research support from NGM Pharmaceuticals. A.C.F. has received grant and research support from Almirall and has acted as a consultant and speaker for Almirall, Norgine and Shire Pharmaceuticals. S.S.R. serves on the advisory board for Forest Labs, Salix Pharmaceuticals, Takeda Pharmaceuticals and Synergy Pharmaceuticals and has received research grants from Forest Labs and Medtronic Corporation. M.S. has received grant and research support from Danone Nutricia Research and Ferring Pharmaceuticals and has acted as a consultant and speaker for Allergan, Almirall, Danone Nutricia Research, Menarini, Nestlé, Shire Pharmaceuticals, Takeda Pharmaceuticals and Tillotts. L.C. has served on advisory boards for BioAmerica, Cairn Diagnostics, IM Healthcare Science LLC, Napo Pharmaceuticals, Salix Pharmaceuticals, and Synergy Pharmaceuticals. All other authors have no relevant conflicts of interest related to the content of this Primer to declare.
Rights and permissions
About this article
Cite this article
Camilleri, M., Ford, A., Mawe, G. et al. Chronic constipation. Nat Rev Dis Primers 3, 17095 (2017). https://doi.org/10.1038/nrdp.2017.95
Published:
DOI: https://doi.org/10.1038/nrdp.2017.95
This article is cited by
-
Physical and psychological correlates of somatic symptom in patients with functional constipation: a cross-sectional study
BMC Psychiatry (2024)
-
Real-world evidence of constipation and laxative use in the Korean population with chronic kidney disease from a common data model
Scientific Reports (2024)
-
Effects of natural products on functional constipation: analysis of active ingredient and mechanism
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Total Flavonoids of Aurantii Fructus Immaturus Regulate miR-5100 to Improve Constipation by Targeting Fzd2 to Alleviate Calcium Balance and Autophagy in Interstitial Cells of Cajal
Molecular Neurobiology (2024)
-
Relationship of Age and Gender to Motility Test Results and Symptoms in Patients with Chronic Constipation
Digestive Diseases and Sciences (2024)