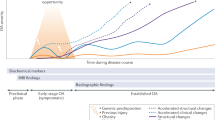
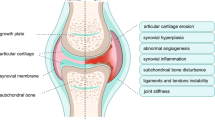
Abstract
Osteoarthritis (OA) is the most common joint disorder, is associated with an increasing socioeconomic impact owing to the ageing population and mainly affects the diarthrodial joints. Primary OA results from a combination of risk factors, with increasing age and obesity being the most prominent. The concept of the pathophysiology is still evolving, from being viewed as cartilage-limited to a multifactorial disease that affects the whole joint. An intricate relationship between local and systemic factors modulates its clinical and structural presentations, leading to a common final pathway of joint destruction. Pharmacological treatments are mostly related to relief of symptoms and there is no disease-modifying OA drug (that is, treatment that will reduce symptoms in addition to slowing or stopping the disease progression) yet approved by the regulatory agencies. Identifying phenotypes of patients will enable the detection of the disease in its early stages as well as distinguish individuals who are at higher risk of progression, which in turn could be used to guide clinical decision making and allow more effective and specific therapeutic interventions to be designed. This Primer is an update on the progress made in the field of OA epidemiology, quality of life, pathophysiological mechanisms, diagnosis, screening, prevention and disease management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Heberden, W. Commentaries on the History and Cure of Diseases (ed. Payne, T. ) (London, 1802).
Garrod, A. B. The Nature and Treatment of Gout and Rheumatic Gout (Walton and Maberly, 1859).
Arden, N. & Cooper, C. in Osteoarthritis Handbook (eds Arden, N. & Cooper, C. ) 1–22 (Taylor and Francis, 2006).
Sellam, J. & Berenbaum, F. Is osteoarthritis a metabolic disease? Joint Bone Spine 80, 568–573 (2013).
Flores, R. H. & Hochberg, M. C. in Osteoarthritis (eds Brandt, K. D., Doherty, M. & Lohmander, L. S. ) 1–8 (Oxford Univ. Press, 2003).
Martel-Pelletier, J., Wildi, L. M. & Pelletier, J.-P. Future therapeutics for osteoarthritis. Bone 51, 297–311 (2012).
Spector, T. D. & Cooper, C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthritis Cartilage 1, 203–206 (1993).
Pelletier, J.-P. et al. What is the predictive value of MRI for the occurrence of knee replacement surgery in knee osteoarthritis? Ann. Rheum. Dis. 72, 1594–1604 (2013).
Felson, D. T. in Osteoarthritis (eds Brandt, K. D., Doherty, M. & Lohmander, L. S. ) 9–16 (Oxford Univ. Press, 2003).
Felson, D. T. et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 30, 914–918 (1987).
van Saase, J. L., van Romunde, L. K., Cats, A., Vandenbroucke, J. P. & Valkenburg, H. A. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann. Rheum. Dis. 48, 271–280 (1989).
Oliveria, S. A., Felson, D. T., Reed, J. I., Cirillo, P. A. & Walker, A. M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 38, 1134–1141 (1995).
Prieto-Alhambra, D. et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 73, 1659–1664 (2014).
Cooper, C. et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 43, 995–1000 (2000).
Cooper, C. et al. Generalized osteoarthritis in women: pattern of joint involvement and approaches to definition for epidemiological studies. J. Rheumatol. 23, 1938–1942 (1996).
Johnson, V. L. & Hunter, D. J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 28, 5–15 (2014).
Palazzo, C., Nguyen, C., Lefevre-Colau, M. M., Rannou, F. & Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 59, 134–138 (2016).
Zhang, Y. et al. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res. (Hoboken) 62, 1527–1532 (2010).
Veronese, N. et al. Osteoarthritis and mortality: a prospective cohort study and systematic review with meta-analysis. Semin. Arthritis Rheum.http://dx.doi.org/10.1016/j.semarthrit.2016.04.002 (2016).
Yusuf, E. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann. Rheum. Dis. 69, 761–765 (2010).
Felson, D. T., Goggins, J., Niu, J., Zhang, Y. & Hunter, D. J. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 50, 3904–3909 (2004).
Ding, C., Stannus, O., Cicuttini, F., Antony, B. & Jones, G. Body fat is associated with increased and lean mass with decreased knee cartilage loss in older adults: a prospective cohort study. Int. J. Obes. (Lond.). 37, 822–827 (2013).
Wang, Y. et al. Relationship between body adiposity measures and risk of primary knee and hip replacement for osteoarthritis: a prospective cohort study. Arthritis Res. Ther. 11, R31 (2009).
Karlson, E. W. et al. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am. J. Med. 114, 93–98 (2003).
Jones, G., Ding, C., Glisson, M., Hynes, K. & Ma, D. Cartilage development in children: a longitudinal study of the effect of sex, growth, body composition and physical activity. Pediatr. Res. 54, 230–236 (2003).
Vanwanseele, B., Eckstein, F., Knecht, H., Spaepen, A. & Stussi, E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthritis Rheum. 48, 3377–3381 (2003).
Wang, Y. et al. Is physical activity a risk factor for primary knee or hip replacement due to osteoarthritis? A prospective cohort study. J. Rheumatol. 38, 350–357 (2011).
Racunica, T. et al. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis Rheum. 57, 1261–1268 (2007).
Teichtahl, A. J. et al. The interaction between physical activity and amount of baseline knee cartilage. Rheumatology (Oxford) 55, 1277–1284 (2016).
Dore, D. A. et al. The association between objectively measured physical activity and knee structural change using MRI. Ann. Rheum. Dis. 72, 1170–1175 (2013). The data of this study demonstrate that the influence of physical activity on joint structure is dependent on the underlying health of the knee joint.
Teichtahl, A. J. et al. Effect of long-term vigorous physical activity on healthy adult knee cartilage. Med. Sci. Sports Exerc. 44, 985–992 (2012).
Timmler, T., Wierusz-Kozlowska, M., Wozniak, W., Markuszewski, J. & Lempicki, A. Development and remodeling of the hip joint of preterm neonates in sonographic evaluation. Ortop. Traumatol. Rehabil. 5, 703–711 (2003).
Nicholls, A. S. et al. The association between hip morphology parameters and nineteen-year risk of end-stage osteoarthritis of the hip: a nested case–control study. Arthritis Rheum. 63, 3392–3400 (2011).
Hussain, S. M. et al. Association of low birth weight and preterm birth with the incidence of knee and hip arthroplasty for osteoarthritis. Arthritis Care Res. (Hoboken) 67, 502–508 (2015).
Agricola, R. et al. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK). Ann. Rheum. Dis. 72, 918–923 (2013).
Siebenrock, K. A. et al. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin. Orthop. Relat. Res. 469, 3229–3240 (2011).
Siebenrock, K. A., Kaschka, I., Frauchiger, L., Werlen, S. & Schwab, J. M. Prevalence of cam-type deformity and hip pain in elite ice hockey players before and after the end of growth. Am. J. Sports Med. 41, 2308–2313 (2013).
Siebenrock, K. A., Behning, A., Mamisch, T. C. & Schwab, J. M. Growth plate alteration precedes cam-type deformity in elite basketball players. Clin. Orthop. Relat. Res. 471, 1084–1091 (2013).
Agricola, R. et al. A cam deformity is gradually acquired during skeletal maturation in adolescent and young male soccer players: a prospective study with minimum 2-year follow-up. Am. J. Sports Med. 42, 798–806 (2014).
Jensen, L. K. Hip osteoarthritis: influence of work with heavy lifting, climbing stairs or ladders, or combining kneeling/squatting with heavy lifting. Occup. Environ. Med. 65, 6–19 (2008).
Teichtahl, A. J. et al. Occupational risk factors for hip osteoarthritis are associated with early hip structural abnormalities: a 3.0 T magnetic resonance imaging study of community-based adults. Arthritis Res. Ther. 17, 19 (2015).
Spector, T. D., Cicuttini, F., Baker, J., Loughlin, J. & Hart, D. Genetic influences on osteoarthritis in women: a twin study. BMJ 312, 940–943 (1996).
Bijkerk, C. et al. Heritabilities of radiologic osteoarthritis in peripheral joints and of disc degeneration of the spine. Arthritis Rheum. 42, 1729–1735 (1999).
Zhai, G. et al. The genetic contribution to muscle strength, knee pain, cartilage volume, bone size, and radiographic osteoarthritis: a sibpair study. Arthritis Rheum. 50, 805–810 (2004).
Rodriguez-Fontenla, C. et al. Assessment of osteoarthritis candidate genes in a meta-analysis of nine genome-wide association studies. Arthritis Rheumatol. 66, 940–949 (2014).
Valdes, A. M. & Spector, T. D. Genetic epidemiology of hip and knee osteoarthritis. Nat. Rev. Rheumatol. 7, 23–32 (2011).
Tsezou, A. Osteoarthritis year in review 2014: genetics and genomics. Osteoarthritis Cartilage 22, 2017–2024 (2014).
Goldring, S. R. & Goldring, M. B. in Kelly's Textbook of Rheumatology (eds Firestein, G. S., Budd, R. C., Gabriel, S. E., McInnes, I. B. & O'Dell, J. R. ) 1–19 (Saunders, 2013).
Heinegard, D. & Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 7, 50–56 (2011). This is a comprehensive overview of the composition of articular cartilage and the molecular and cellular changes that are associated with OA.
Houard, X., Goldring, M. B. & Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 15, 375 (2013).
Quinn, T. M., Hauselmann, H. J., Shintani, N. & Hunziker, E. B. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthritis Cartilage 21, 1904–1912 (2013).
Andriacchi, T. P. & Favre, J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr. Rheumatol. Rep. 16, 463 (2014).
Guo, H., Maher, S. A. & Torzilli, P. A. A biphasic finite element study on the role of the articular cartilage superficial zone in confined compression. J. Biomech. 48, 166–170 (2015).
Wilusz, R. E., Sanchez-Adams, J. & Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 39, 25–32 (2014).
Loeser, R. F. Integrins and chondrocyte–matrix interactions in articular cartilage. Matrix Biol. 39, 11–16 (2014).
Xu, L., Golshirazian, I., Asbury, B. J. & Li, Y. Induction of high temperature requirement A1, a serine protease, by TGF-β1 in articular chondrocytes of mouse models of OA. Histol. Histopathol. 29, 609–618 (2014).
Maes, C. et al. VEGF-independent cell-autonomous functions of HIF-1α regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J. Bone Miner. Res. 27, 596–609 (2012).
Ruhlen, R. & Marberry, K. The chondrocyte primary cilium. Osteoarthritis Cartilage 22, 1071–1076 (2014).
Burr, D. B. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage 12, S20–S30 (2004).
Burr, D. B. & Schaffler, M. B. The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Microsc. Res. Tech. 37, 343–357 (1997).
Imhof, H. et al. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest. Radiol. 35, 581–588 (2000).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 49, 1852–1861 (2010).
Suri, S. & Walsh, D. A. Osteochondral alterations in osteoarthritis. Bone 51, 204–211 (2012).
Lohmander, L. S., Saxne, T. & Heinegard, D. K. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann. Rheum. Dis. 53, 8–13 (1994).
Fosang, A. J. & Beier, F. Emerging frontiers in cartilage and chondrocyte biology. Best Pract. Res. Clin. Rheumatol. 25, 751–766 (2011).
Wang, M. et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 15, R5 (2013).
Marcu, K. B., Otero, M., Olivotto, E., Borzi, R. M. & Goldring, M. B. NF-κB signaling: multiple angles to target OA. Curr. Drug Targets 11, 599–613 (2010).
Goldring, M. B. et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell. Mater. 21, 202–220 (2011).
Cuervo, A. M. & Wong, E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 24, 92–104 (2014).
Liu-Bryan, R. & Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11, 35–44 (2015). This is an overview of the molecular mechanisms that are involved in joint inflammation in OA.
Loeser, R. F. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 17, 971–979 (2009).
Sandell, L. J. Etiology of osteoarthritis: genetics and synovial joint development. Nat. Rev. Rheumatol. 8, 77–89 (2012).
Eyre, D. R. Collagens and cartilage matrix homeostasis. Clin. Orthop. Relat. Res. 427, S118–S122 (2004).
Goldring, S. R. Role of bone in osteoarthritis pathogenesis. Med. Clin. North Am. 93, 25–35 (2009).
Goldring, M. B. & Goldring, S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. NY Acad. Sci. 1192, 230–237 (2010).
Burr, D. B. & Gallant, M. A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8, 665–673 (2012). This is an overview of OA bone pathology and the role of bone in OA pathogenesis.
Goldring, S. R. The role of bone in osteoarthritis pathogenesis. Rheum. Dis. Clin. North Am. 34, 561–571 (2008).
Raynauld, J. P. et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann. Rheum. Dis. 67, 683–688 (2008).
Ko, F. C. et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 65, 1569–1578 (2013).
Brown, T. D., Radin, E. L., Martin, R. B. & Burr, D. B. Finite element studies of some juxtarticular stress changes due to localized subchondral stiffening. J. Biomech. 17, 11–24 (1984).
Poulet, B., Hamilton, R. W., Shefelbine, S. & Pitsillides, A. A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 63, 137–147 (2011).
Radin, E. L. & Rose, R. M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 213, 34–40 (1986).
Day, J. S. et al. Adaptation of subchondral bone in osteoarthritis. Biorheology 41, 359–368 (2004).
Day, J. S. et al. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J. Orthop. Res. 19, 914–918 (2001).
Taljanovic, M. S. et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol. 37, 423–431 (2008).
Leydet-Quilici, H. et al. Advanced hip osteoarthritis: magnetic resonance imaging aspects and histopathology correlations. Osteoarthritis Cartilage 18, 1429–1435 (2010).
Bowes, M. A. et al. Osteoarthritic bone marrow lesions almost exclusively colocate with denuded cartilage: a 3D study using data from the Osteoarthritis Initiative. Ann. Rheum. Dis. 75, 1852–1857 (2016).
Crema, M. D. et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: detection with MR imaging — the MOST study. Radiology 256, 855–862 (2010).
van der Kraan, P. M. & van den Berg, W. B. Osteophytes: relevance and biology. Osteoarthritis Cartilage 15, 237–244 (2007).
Pottenger, L. A., Phillips, F. M. & Draganich, L. F. The effect of marginal osteophytes on reduction o varus-valgus instability in osteoarthritic knees. Arthritis Rheum. 33, 853–858 (1990).
Felson, D. T. et al. Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxford) 44, 100–104 (2005).
Scanzello, C. R. & Goldring, S. R. The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257 (2012).
Baker, K. et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 69, 1779–1783 (2010).
Felson, D. T. et al. Synovitis and the risk of knee osteoarthritis: the MOST study. Osteoarthritis Cartilage 24, 458–464 (2016).
Roemer, F. W. et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809 (2011).
Pelletier, J.-P. et al. A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage 16, S8–S13 (2008).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 (2012).
Ritter, S. Y. et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 65, 981–992 (2013).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J.-P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011).
Scanzello, C. R. et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage 17, 1040–1048 (2009).
Cuellar, J. M., Scuderi, G. J., Cuellar, V. G., Golish, S. R. & Yeomans, D. C. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J. Bone Joint Surg. Am. 91, 2313–2320 (2009).
Scanzello, C. R. et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 63, 391–400 (2011).
Takebe, K., Rai, M. F., Schmidt, E. J. & Sandell, L. J. The chemokine receptor CCR5 plays a role in post-traumatic cartilage loss in mice, but does not affect synovium and bone. Osteoarthritis Cartilage 23, 454–461 (2015).
Wang, X., Hunter, D., Xu, J. & Ding, C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage 23, 22–30 (2015).
Stannus, O. P. et al. Cross-sectional and longitudinal associations between circulating leptin and knee cartilage thickness in older adults. Ann. Rheum. Dis. 74, 82–88 (2015).
Martel-Pelletier, J., Raynauld, J. P., Dorais, M., Abram, F. & Pelletier, J.-P. The levels of the adipokines adipsin and leptin are associated with knee osteoarthritis progression as assessed by MRI and incidence of total knee replacement in symptomatic osteoarthritis patients: a post hoc analysis. Rheumatology (Oxford) 55, 680–688 (2016).
Teichtahl, A. J. et al. Vastus medialis fat infiltration — a modifiable determinant of knee cartilage loss. Osteoarthritis Cartilage 23, 2150–2157 (2015). This paper shows that fat atrophy of the quadriceps, which can be modified, is associated with structural changes in knee cartilage. Intramuscular fat may be a therapeutic target for altering the natural history of knee OA.
Raynauld, J. P. et al. Magnetic resonance imaging-assessed vastus medialis muscle fat content and risk for knee osteoarthritis progression: relevance from a clinical trial. Arthritis Care Res. (Hoboken) 67, 1406–1415 (2015). In addition to showing that vastus medialis fat content is a strong predictor of cartilage volume loss, this study demonstrates that combining vastus medialis area and fat content and BMI identified patients who are at higher risk for OA progression.
Wang, Y. et al. Increase in vastus medialis cross-sectional area is associated with reduced pain, cartilage loss, and joint replacement risk in knee osteoarthritis. Arthritis Rheum. 64, 3917–3925 (2012).
Varady, N. H. & Grodzinsky, A. J. Osteoarthritis year in review 2015: mechanics. Osteoarthritis Cartilage 24, 27–35 (2016).
Bennell, K. L. et al. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann. Rheum. Dis. 70, 1770–1774 (2011).
Donahue, T. L., Fisher, M. B. & Maher, S. A. Meniscus mechanics and mechanobiology. J. Biomech. 48, 1341–1342 (2015).
Courties, A., Gualillo, O., Berenbaum, F. & Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage 23, 1955–1965 (2015).
Altman, R. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 29, 1039–1049 (1986).
Wu, C. W. et al. Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Semin. Arthritis Rheum. 35, 197–201 (2005).
Altman, R. et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 33, 1601–1610 (1990).
Altman, R. et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 34, 505–514 (1991).
Guermazi, A. et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ 345, e5339 (2012).
Schiphof, D. et al. Crepitus is a first indication of patellofemoral osteoarthritis (and not of tibiofemoral osteoarthritis). Osteoarthritis Cartilage 22, 631–638 (2014).
Ike, R. & O'Rourke, K. S. Compartment-directed physical examination of the knee can predict articular cartilage abnormalities disclosed by needle arthroscopy. Arthritis Rheum. 38, 917–925 (1995).
Jones, G. What's new in osteoarthritis pathogenesis? Intern. Med. J. 46, 229–236 (2016).
Zhai, G. et al. Correlates of knee pain in older adults: Tasmanian Older Adult Cohort Study. Arthritis Rheum. 55, 264–271 (2006).
Bedson, J. & Croft, P. R. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet. Disord. 9, 116 (2008).
Hunter, D. J. et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage 19, 963–969 (2011).
Haugen, I. K. et al. Associations between MRI-defined synovitis, bone marrow lesions and structural features and measures of pain and physical function in hand osteoarthritis. Ann. Rheum. Dis. 71, 899–904 (2012).
Ahedi, H., Aitken, D., Blizzard, L., Cicuttini, F. & Jones, G. A population-based study of the association between hip bone marrow lesions, high cartilage signal, and hip and knee pain. Clin. Rheumatol. 33, 369–376 (2014).
Felson, D. T., Zhang, Y., Anthony, J. M., Naimark, A. & Anderson, J. J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann. Intern. Med. 116, 535–539 (1992).
Teichtahl, A. J. et al. Weight change and change in tibial cartilage volume and symptoms in obese adults. Ann. Rheum. Dis. 74, 1024–1029 (2015).
Wang, Y. et al. Body composition and knee cartilage properties in healthy, community-based adults. Ann. Rheum. Dis. 66, 1244–1248 (2007).
Fernandes, L. et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 72, 1125–1135 (2013).
Belo, J. N., Berger, M. Y., Reijman, M., Koes, B. W. & Bierma-Zeinstra, S. M. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 57, 13–26 (2007).
Christensen, R., Bartels, E. M., Astrup, A. & Bliddal, H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 66, 433–439 (2007).
Fransen, M. et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 1, CD004376 (2015).
Fransen, M., McConnell, S., Hernandez-Molina, G. & Reichenbach, S. Exercise for osteoarthritis of the hip. Cochrane Database Syst. Rev. 4, CD007912 (2014).
Ruhdorfer, A., Wirth, W. & Eckstein, F. Longitudinal change in thigh muscle strength prior to and concurrent with minimum clinically important worsening or improvement in knee function: data from the Osteoarthritis Initiative. Arthritis Rheumatol. 68, 826–836 (2016).
Hunter, D. J. et al. The intensive diet and exercise for arthritis (IDEA) trial: 18-month radiographic and MRI outcomes. Osteoarthritis Cartilage 23, 1090–1098 (2015).
Messier, S. P. et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 310, 1263–1273 (2013).
Atukorala, I. et al. Is there a dose–response relationship between weight loss and symptom improvement in persons with knee osteoarthritis? Arthritis Care Res. (Hoboken) 68, 1106–1114 (2016). This study highlights the importance of weight loss in the treatment of OA by demonstrating a dose–response effect between percentage weight loss and improvement of symptoms in a cohort of obese individuals with symptomatic knee OA.
Rannou, F. et al. Splint for base-of-thumb osteoarthritis: a randomized trial. Ann. Intern. Med. 150, 661–669 (2009).
Gomes Carreira, A. C., Jones, A. & Natour, J. Assessment of the effectiveness of a functional splint for osteoarthritis of the trapeziometacarpal joint on the dominant hand: a randomized controlled study. J. Rehabil. Med. 42, 469–474 (2010).
Jones, A. et al. Impact of cane use on pain, function, general health and energy expenditure during gait in patients with knee osteoarthritis: a randomised controlled trial. Ann. Rheum. Dis. 71, 172–179 (2012).
Callaghan, M. J. et al. A randomised trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann. Rheum. Dis. 74, 1164–1170 (2015). This study identifies the efficacy of a knee brace in the treatment of patellofemoral OA, in which it conferred structural and symptomatic benefits by reducing the size of bone marrow lesions and knee pain.
Palmer, S. et al. Transcutaneous electrical nerve stimulation as an adjunct to education and exercise for knee osteoarthritis: a randomized controlled trial. Arthritis Care Res. (Hoboken) 66, 387–394 (2014).
Laufer, Y. & Dar, G. Effectiveness of thermal and athermal short-wave diathermy for the management of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 20, 957–966 (2012).
Zhang, W. et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18, 476–499 (2010).
Machado, G. C. et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 350, h1225 (2015).
da Costa, B. R. et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 387, 2093–2105 (2016).
Roberts, E. et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann. Rheum. Dis. 75, 552–559 (2016).
Hochberg, M. C. et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. (Hoboken) 64, 465–474 (2012).
National Institute for Health and Care Excellence. Osteoarthritis care and management in adults. NICEhttps://www.nice.org.uk/Guidance/cg177 (2014).
McAlindon, T. E. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22, 363–388 (2014).
Singh, J. A., Noorbaloochi, S., MacDonald, R. & Maxwell, L. J. Chondroitin for osteoarthritis. Cochrane Database Syst. Rev. 1, CD005614 (2015).
Towheed, T. E. et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst. Rev. 18, CD002946 (2005).
Bruyere, O. et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 44, 253–263 (2014).
Hochberg, M. C. et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann. Rheum. Dis. 75, 37–44 (2016).
Martel-Pelletier, J. et al. First-line analysis of the effects of treatment on progression of structural changes in knee osteoarthritis over 24 months: data from the osteoarthritis initiative progression cohort. Ann. Rheum. Dis. 74, 547–556 (2015).
Fransen, M. et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann. Rheum. Dis. 74, 851–858 (2015).
Gaffney, K., Ledingham, J. & Perry, J. D. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann. Rheum. Dis. 54, 379–381 (1995).
Arden, N. K. et al. A randomised controlled trial of tidal irrigation versus corticosteroid injection in knee osteoarthritis: the KIVIS study. Osteoarthritis Cartilage 16, 733–739 (2008).
Godwin, M. & Dawes, M. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Can. Fam. Physician 50, 241–248 (2004).
Rutjes, A. W. et al. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann. Intern. Med. 157, 180–191 (2012).
Lewis, G. N., Rice, D. A., McNair, P. J. & Kluger, M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br. J. Anaesth. 114, 551–561 (2015).
Santaguida, P. L. et al. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can. J. Surg. 51, 428–436 (2008).
Wainwright, C., Theis, J. C., Garneti, N. & Melloh, M. Age at hip or knee joint replacement surgery predicts likelihood of revision surgery. J. Bone Joint Surg. Br. 93, 1411–1415 (2011).
Kahan, A., Uebelhart, D., De Vathaire, F., Delmas, P. D. & Reginster, J. Y. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 60, 524–533 (2009).
Reginster, J. Y. et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 357, 251–256 (2001).
Wildi, L. M. et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann. Rheum. Dis. 70, 982–989 (2011).
Pelletier, J.-P. et al. Disease-modifying effect of strontium ranelate in a subset of patients from the phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann. Rheum. Dis. 74, 422–429 (2015).
Medicines and Healthcare products Regulatory Agency. Strontium ranelate: cardiovascular risk. GOV.UKhttps://www.gov.uk/drug-safety-update/strontium-ranelate-cardiovascular-risk (accessed 09 August 2016).
Dennison, E. & Cooper, C. in Textbook of Osteoarthritis (eds Brandt, K. D., Doherty, M. & Lohmander, L. S. ) 227–223 (Oxford Univ. Press, 2003).
Roos, E. M. Hip dysfunction and osteoarthritis outcome score (HOOS). KOOShttp://www.koos.nu (accessed 21 June 2016).
Roos, E. M. & Lohmander, L. S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual. Life Outcomes 1, 64 (2003).
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196 (2012).
Cross, M. et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73, 1323–1330 (2014).
Glyn-Jones, S. et al. Osteoarthritis. Lancet 386, 376–387 (2015).
Woolf, A. D. & Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 81, 646–656 (2003).
Hiligsmann, M. et al. Health economics in the field of osteoarthritis: an expert's consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 43, 303–313 (2013).
Litwic, A., Edwards, M. H., Dennison, E. M. & Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 105, 185–199 (2013).
Palazzo, C., Ravaud, J. F., Papelard, A., Ravaud, P. & Poiraudeau, S. The burden of musculoskeletal conditions. PLoS ONE 9, e90633 (2014).
Cooper, C. & Arden, N. K. Excess mortality in osteoarthritis. BMJ 342, d1407 (2011).
Nuesch, E. et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 342, d1165 (2011).
Hawker, G. A. et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS ONE 9, e91286 (2014).
Barbour, K. E. et al. Hip osteoarthritis and the risk of all-cause and disease-specific mortality in older women: a population-based cohort study. Arthritis Rheumatol. 67, 1798–1805 (2015).
Uhlig, T., Slatkowsky-Christensen, B., Moe, R. H. & Kvien, T. K. The burden of osteoarthritis: the societal and the patient perspective. Therapy 7, 605–619 (2010).
Hochberg, M. C. Mortality in osteoarthritis. Clin. Exp. Rheumatol. 26, S120–S124 (2008).
Pelletier, J.-P. & Martel-Pelletier, J. The DMOAD dream: a generation later. Rheumatologist 5, 18–20 (2011).
Arden, N. et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on biomarkers and frailty. Drugs Aging 32, 525–535 (2015).
Roubille, C., Pelletier, J.-P. & Martel-Pelletier, J. in Osteoarthritis: Pathogenesis, Diagnosis, Available Treatments, Drug Safety, Regenerative and Precision Medicine (eds Kapoor, M. & Mahomed, N. N. ) 191–210 (Springer International Publishing, 2015).
Berenbaum, F., Pelletier, J.-P. & Burkhard, L. Osteoarthritis: the challenge of establishing a personalised treatment. EMJ Rheumatol. 1, 33–39 (2014).
Roubille, C., Pelletier, J.-P. & Martel-Pelletier, J. New and emerging treatments for osteoarthritis management: will the dream come true with personalized medicine? Expert Opin. Pharmacother. 14, 2059–2077 (2013).
Cooper, C. et al. How to define responders in osteoarthritis. Curr. Med. Res. Opin. 29, 719–729 (2013).
Peterfy, C. G., Schneider, E. & Nevitt, M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 16, 1433–1441 (2008).
Ding, C. et al. Association between leptin, body composition, sex and knee cartilage morphology in older adults: the Tasmanian older adult cohort (TASOAC) study. Ann. Rheum. Dis. 67, 1256–1261 (2008).
Solomon, D. H. et al. The comparative safety of analgesics in older adults with arthritis. Arch. Intern. Med. 170, 1968–1976 (2010).
Conaghan, P. G., Hunter, D. J., Maillefert, J. F., Reichmann, W. M. & Losina, E. Summary and recommendations of the OARSI FDA osteoarthritis Assessment of Structural Change Working Group. Osteoarthritis Cartilage 19, 606–610 (2011).
Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell… and more. Endocr. Rev. 34, 658–690 (2013).
Zhang, W. et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 69, 483–489 (2010).
Zhang, W. et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann. Rheum. Dis. 68, 8–17 (2009).
Barr, A. & Conaghan, P. G. Osteoarthritis: recent advances in diagnosis and management. Prescriber 25, 26–33 (2014).
Acknowledgements
J.M.-P. and J.-P.P. acknowledge the Chair in Osteoarthritis of the University of Montreal and the Groupe de recherche des maladies rhumatismales du Québec for their support in osteoarthritis research. The authors thank V. Wallis for her assistance with the manuscript preparation.
Author information
Authors and Affiliations
Contributions
Introduction (J.M.-P. and J.-P.P.); Epidemiology (C.C.); Mechanisms/pathophysiology (M.B.G. and S.R.G.); Diagnosis, screening and prevention (F.M.C., G.J. and A.J.T.); Management (A.J.B. and P.G.C.); Quality of life (C.C.); Outlook (J.M.-P. and J.-P.P.); Overview of Primer (J.M.-P. and J.-P.P.).
Corresponding authors
Ethics declarations
Competing interests
J.M.-P. is a shareholder of ArthroLab Inc. and consultant for AbbVie, Bioibrica, Ferring, Medapharma, Pierre-Fabre and TRB Chemedica. P.G.C. is a member of the speakers bureau and/or is a consultant for AbbVie, Flexion, Janssen, Lilly, Novartis, Pfizer, Regeneron and Roche. C.C. has received consultancy fees and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GlasoSmithKline, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB. J.-P.P. is a shareholder of ArthroLab Inc. and is a consultant for AbbVie, Bioibrica, Centrexion, Ferring, Medapharma, Pfizer, Pierre-Fabre, Teva Pharmaceuticals and TRB Chemedica. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Martel-Pelletier, J., Barr, A., Cicuttini, F. et al. Osteoarthritis. Nat Rev Dis Primers 2, 16072 (2016). https://doi.org/10.1038/nrdp.2016.72
Published:
DOI: https://doi.org/10.1038/nrdp.2016.72
This article is cited by
-
Calycosin ameliorates osteoarthritis by regulating the imbalance between chondrocyte synthesis and catabolism
BMC Complementary Medicine and Therapies (2024)
-
Klf10 is involved in extracellular matrix calcification of chondrocytes alleviating chondrocyte senescence
Journal of Translational Medicine (2024)
-
Glaucocalyxin A delays the progression of OA by inhibiting NF-κB and MAPK signaling pathways
Journal of Orthopaedic Surgery and Research (2024)
-
ADAM8 silencing suppresses the migration and invasion of fibroblast-like synoviocytes via FSCN1/MAPK cascade in osteoarthritis
Arthritis Research & Therapy (2024)
-
Clinical applications of stem cell-derived exosomes
Signal Transduction and Targeted Therapy (2024)