Abstract
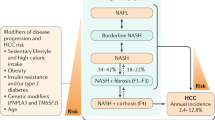
Nonalcoholic fatty liver disease (NAFLD) is a disorder characterized by excess accumulation of fat in hepatocytes (nonalcoholic fatty liver (NAFL)); in up to 40% of individuals, there are additional findings of portal and lobular inflammation and hepatocyte injury (which characterize nonalcoholic steatohepatitis (NASH)). A subset of patients will develop progressive fibrosis, which can progress to cirrhosis. Hepatocellular carcinoma and cardiovascular complications are life-threatening co-morbidities of both NAFL and NASH. NAFLD is closely associated with insulin resistance; obesity and metabolic syndrome are common underlying factors. As a consequence, the prevalence of NAFLD is estimated to be 10–40% in adults worldwide, and it is the most common liver disease in children and adolescents in developed countries. Mechanistic insights into fat accumulation, subsequent hepatocyte injury, the role of the immune system and fibrosis as well as the role of the gut microbiota are unfolding. Furthermore, genetic and epigenetic factors might explain the considerable interindividual variation in disease phenotype, severity and progression. To date, no effective medical interventions exist that completely reverse the disease other than lifestyle changes, dietary alterations and, possibly, bariatric surgery. However, several strategies that target pathophysiological processes such as an oversupply of fatty acids to the liver, cell injury and inflammation are currently under investigation. Diagnosis of NAFLD can be established by imaging, but detection of the lesions of NASH still depend on the gold-standard but invasive liver biopsy. Several non-invasive strategies are being evaluated to replace or complement biopsies, especially for follow-up monitoring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910 (2014).
Brunt, E. M. Non-alcoholic fatty liver disease: what's new under the microscope? Gut 60, 1152–1158 (2011). This article provides a detailed description of the histological lesions of NAFLD and NASH and their correlations with pathophysiology and the clinical features of disease.
Torres, D. M. & Harrison, S. A. Nonalcoholic fatty liver disease: fibrosis portends a worse prognosis. Hepatology 61, 1462–1464 (2015).
Adams, L. A. et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129, 113–121 (2005).
Ekstedt, M. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015).
Baffy, G., Brunt, E. M. & Caldwell, S. H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J. Hepatol. 56, 1384–1391 (2012).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397 (2015).
Harmon, R. C., Tiniakos, D. G. & Argo, C. K. Inflammation in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 5, 189–200 (2011).
Rinella, M. E. Nonalcoholic fatty liver disease. JAMA 313, 2263–2273 (2015).
Araújo, L. M., De Oliveira, D. A. & Nunes, D. S. Liver and biliary ultrasonography in diabetic and non-diabetic obese women. Diabetes Metab. 24, 458–462 (1998).
Bedogni, G. et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 42, 44–52 (2005).
Williams, C. D. et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140, 124–131 (2011).
Zelber-Sagi, S., Nitzan-Kaluski, D., Halpern, Z. & Oren, R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 26, 856–863 (2006).
Browning, J. D. et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40, 1387–1395 (2004).
Targher, G. et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30, 1212–1218 (2007).
Wanless, I. R. & Lentz, J. S. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 12, 1106–1110 (1990).
Silverman, J. F. et al. Liver pathology in morbidly obese patients with and without diabetes. Am. J. Gastroenterol. 85, 1349–1355 (1990).
Matteoni, C. A. et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116, 1413–1419 (1999).
Dixon, J. B., Bhathal, P. S. & O'Brien, P. E. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 121, 91–100 (2001).
Hyysalo, J. et al. A population-based study on the prevalence of NASH using scores validated against liver histology. J. Hepatol. 60, 839–846 (2014).
Ascha, M. S. et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 51, 1972–1978 (2010).
Wong, R. J. et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148, 547–555 (2015). Data from the United Network for Organ Sharing/Organ Procurement and Transplantation Network registry indicate that NASH is the most rapidly growing indication for liver transplantation in the United States in the past 10 years.
Fan, J.-G. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J. Gastroenterol. Hepatol. 28 (Suppl. 1), 11–17 (2013).
Kojima, S.-I., Watanabe, N., Numata, M., Ogawa, T. & Matsuzaki, S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J. Gastroenterol. 38, 954–961 (2003).
Wong, V. W.-S. et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J. Hepatol. 62, 182–189 (2015).
Wong, V. W.-S. et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 61, 409–415 (2012).
Welsh, J. A., Karpen, S. & Vos, M. B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatr. 162, 496–500 (2013).
Mangge, H. et al. Patatin-like phospholipase 3 (rs738409) gene polymorphism is associated with increased liver enzymes in obese adolescents and metabolic syndrome in all ages. Aliment. Pharmacol. Ther. 42, 99–105 (2015).
Nobili, V. et al. Nonalcoholic fatty liver disease. JAMA Pediatr. 169, 170–176 (2015).
Schwimmer, J. B. et al. Prevalence of fatty liver in children and adolescents. Pediatrics 118, 1388–1393 (2006). This was the first study to accurately assess fatty liver in the paediatric population. The study was based on autopsies of individuals with no known liver disease; careful racial and ethnic and gender assessments were made.
Dunn, W. & Schwimmer, J. B. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr. Gastroenterol. Rep. 10, 67–72 (2008).
Schwimmer, J. B. et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology 136, 1585–1592 (2009).
Yang, H. R., Yi, D. Y. & Choi, H. S. Comparison between a pediatric health promotion center and a pediatric obesity clinic in detecting metabolic syndrome and non-alcoholic fatty liver disease in children. J. Korean Med. Sci. 29, 1672–1677 (2014).
Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 686–690 (2013).
Chan, D. F. Y. et al. Hepatic steatosis in obese Chinese children. Int. J. Obes. Relat. Metab. Disord. 28, 1257–1263 (2004).
Booth, M. L. et al. The population prevalence of adverse concentrations and associations with adiposity of liver tests among Australian adolescents. J. Paediatr. Child Health 44, 686–691 (2008).
Maher, J. J., Leon, P. & Ryan, J. C. Beyond insulin resistance: innate immunity in nonalcoholic steatohepatitis. Hepatology 48, 670–678 (2008).
He, J., Lee, J. H., Febbraio, M. & Xie, W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. (Maywood) 236, 1116–1121 (2011).
Mitsuyoshi, H. et al. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol. Res. 39, 366–373 (2009).
Samuel, V. T. et al. Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 117, 739–745 (2007).
Postic, C. & Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118, 829–838 (2008).
Lambert, J. E., Ramos-Roman, M. A., Browning, J. D. & Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735 (2014).
Donnelly, K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005). This paper documents the relative contribution of dietary fat, DNL and adipose tissue lipolysis to hepatic lipid accumulation in humans.
Li, S., Brown, M. S. & Goldstein, J. L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl Acad. Sci. USA 107, 3441–3446 (2010).
Kammoun, H. L. et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 119, 1201–1215 (2009).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Schnabl, B. & Brenner, D. A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524 (2014). This review documents the role of gut dysbiosis in the pathogenesis of NAFLD.
Koliaki, C. et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 21, 739–746 (2015). This paper documents that hepatic steatosis is marked by a compensatory increase in mitochondrial activity, but this compensation is lost as NASH develops.
Begriche, K., Massart, J., Robin, M.-A., Bonnet, F. & Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 58, 1497–1507 (2013).
Luedde, T., Kaplowitz, N. & Schwabe, R. F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147, 765–783 (2014).
Upton, J.-P. et al. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol. Cell. Biol. 28, 3943–3951 (2008).
Johnson, E. S. et al. Metabolomic profiling reveals a role for caspase-2 in lipoapoptosis. J. Biol. Chem. 288, 14463–14475 (2013).
Machado, M. V. et al. Reduced lipoapoptosis, Hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut 64, 1148–1157 (2015).
Feldstein, A. E. et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125, 437–443 (2003).
Cazanave, S. C. et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J. Biol. Chem. 286, 39336–39348 (2011).
Inokuchi-Shimizu, S. et al. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J. Clin. Invest. 124, 3566–3578 (2014).
Gautheron, J. et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol. Med. 6, 1062–1074 (2014).
Wehr, A. et al. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS ONE 9, e112327 (2014).
Syn, W.-K. et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology 51, 1998–2007 (2010).
Syn, W.-K. et al. NKT-associated Hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut 61, 1323–1329 (2012).
Harley, I. T. W. et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 59, 1830–1839 (2014).
Sutti, S. et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 59, 886–897 (2014).
Wolf, M. J. et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564 (2014).
Roh, Y. S. & Seki, E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 28 (Suppl. 1), 38–42 (2013).
Mehal, W. Z. The inflammasome in liver injury and non-alcoholic fatty liver disease. Dig. Dis. 32, 507–515 (2014). This review describes the role of inflammasome activation in the pathogenesis of NAFLD.
Tosello-Trampont, A.-C., Landes, S. G., Nguyen, V., Novobrantseva, T. I. & Hahn, Y. S. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J. Biol. Chem. 287, 40161–40172 (2012).
Leroux, A. et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J. Hepatol. 57, 141–149 (2012).
Wallace, M. C., Friedman, S. L. & Mann, D. A. Emerging and disease-specific mechanisms of hepatic stellate cell activation. Semin. Liver Dis. 35, 107–118 (2015).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Tomita, K. et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 59, 154–169 (2014).
Jiang, J. X. et al. Advanced glycation endproducts induce fibrogenic activity in nonalcoholic steatohepatitis by modulating TNF-α-converting enzyme activity in mice. Hepatology 58, 1339–1348 (2013).
Rangwala, F. et al. Increased production of Sonic Hedgehog by ballooned hepatocytes. J. Pathol. 224, 401–410 (2011).
Choi, S. S., Omenetti, A., Syn, W.-K. & Diehl, A. M. The role of Hedgehog signaling in fibrogenic liver repair. Int. J. Biochem. Cell Biol. 43, 238–244 (2011).
Hirsova, P., Ibrahim, S. H., Bronk, S. F., Yagita, H. & Gores, G. J. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS ONE 8, e70599 (2013).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 (2013).
Al-Serri, A. et al. The SOD2 C47T polymorphism influences NAFLD fibrosis severity: evidence from case–control and intra-familial allele association studies. J. Hepatol. 56, 448–454 (2012).
Dong, H. et al. The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population. J. Hepatol. 46, 915–920 (2007).
Wang, L. et al. Fatty acid desaturase 1 gene polymorphisms control human hepatic lipid composition. Hepatology 61, 119–128 (2015).
Miele, L. et al. The Kruppel-like factor 6 genotype is associated with fibrosis in nonalcoholic fatty liver disease. Gastroenterology 135, 282–291 (2008).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008). This is the first genome-wide association study in NAFLD and convincingly identified PNPLA3 as a risk factor for NAFLD.
Chalasani, N. et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology 139, 1567–1576 (2010).
Speliotes, E. K. et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 7, e1001324 (2011).
Anstee, Q. et al. Genome-wide association analysis confirms importance of PNPLA3 and identifies novel variants associated with histologically progressive steatohepatitis in NAFLD. Hepatology 56, 265A–266A(2012).
Kawaguchi, T. et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS ONE 7, e38322 (2012).
Feitosa, M. F. et al. The ERLIN1–CHUK–CWF19L1 gene cluster influences liver fat deposition and hepatic inflammation in the NHLBI Family Heart study. Atherosclerosis 228, 175–180 (2013).
Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356 (2014). This exome-wide association study identified TM6SF2 as the second gene that confers susceptibility to NAFLD.
DiStefano, J. K. et al. Genome-wide analysis of hepatic lipid content in extreme obesity. Acta Diabetol. 52, 373–382 (2015).
Valenti, L. et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 51, 1209–1217 (2010).
Liu, Y.-L. et al. Carriage of the PNPLA3 rs738409 C>G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 61, 75–81 (2014).
Smagris, E. et al. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 61, 108–118 (2015).
Pirazzi, C. et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum. Mol. Genet. 23, 4077–4085 (2014).
Liu, Y.-L. et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 5, 4309 (2014).
Holmen, O. L. et al. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat. Genet. 46, 345–351 (2014).
Kahali, B. et al. TM6SF2: catch-22 in the fight against nonalcoholic fatty liver disease and cardiovascular disease? Gastroenterology 148, 679–684 (2015).
Dongiovanni, P. et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 61, 506–514 (2015).
Sookoian, S. & Pirola, C. J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 53, 1883–1894 (2011).
Murphy, S. K. et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology 145, 1076–1087 (2013).
Mann, J. et al. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 14, 275–285 (2007).
Kitamoto, T. et al. Targeted-bisulfite sequence analysis of the methylation of CpG islands in genes encoding PNPLA3, SAMM50, and PARVB of patients with non-alcoholic fatty liver disease. J. Hepatol. 63, 494–502 (2015).
Zeybel, M. et al. Differential DNA methylation of genes involved in fibrosis progression in non-alcoholic fatty liver disease and alcoholic liver disease. Clin. Epigenetics 7, 25 (2015).
Sookoian, S. et al. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology 52, 1992–2000 (2010).
Pirola, C. J. et al. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut 62, 1356–1363 (2013). This study provides first evidence of the role of mitochondrial epigenetics in human NAFLD.
Horvath, S. et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl Acad. Sci. USA 111, 15538–15543 (2014).
Pirola, C. J. et al. Epigenetic modifications in the biology of nonalcoholic fatty liver disease: the role of DNA hydroxymethylation and TET proteins. Medicine (Baltimore) 94, e1480 (2015).
Zeybel, M. et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat. Med. 18, 1369–1377 (2012).
Sookoian, S., Gianotti, T. F., Burgueño, A. L. & Pirola, C. J. Fetal metabolic programming and epigenetic modifications: a systems biology approach. Pediatr. Res. 73, 531–542 (2013).
Carr, S. K. et al. Maternal diet amplifies the hepatic aging trajectory of Cidea in male mice and leads to the development of fatty liver. FASEB J. 28, 2191–2201 (2014).
Suter, M. A. et al. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J. 26, 5106–5114 (2012).
Shen, J. et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J. Hepatol. 56, 1363–1370 (2012).
Min, H.-K. et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15, 665–674 (2012).
Pirola, C. J. et al. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut 64, 800–812 (2015).
Esau, C. et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 3, 87–98 (2006).
Hsu, S.-H. et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Invest. 122, 2871–2883 (2012).
Li, J. et al. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 58, 522–528 (2013).
Csak, T. et al. microRNA-122 regulates hypoxia-inducible factor-1 and vimentin in hepatocytes and correlates with fibrosis in diet-induced steatohepatitis. Liver Int. 35, 532–541 (2015).
Takaki, Y. et al. Silencing of microRNA-122 is an early event during hepatocarcinogenesis from non-alcoholic steatohepatitis. Cancer Sci. 105, 1254–1260 (2014).
Wu, Q. et al. Metabolic phenotype-microRNA data fusion analysis of the systemic consequences of Roux-en-Y gastric bypass surgery. Int. J. Obes. (Lond.) 39, 1126–1134 (2015).
Ahrens, M. et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 18, 296–302 (2013). This study shows the plasticity of the liver epigenome, which can be modulated by therapeutic intervention.
Li, P. et al. A liver-enriched long non-coding RNA, lncLSTR, regulates systemic lipid metabolism in mice. Cell Metab. 21, 455–467 (2015).
Vassilatou, E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J. Gastroenterol. 20, 8351–8363 (2014).
Hossain, N. et al. Non-alcoholic steatohepatitis (NASH) in patients with polycystic ovarian syndrome (PCOS). Scand. J. Gastroenterol. 46, 479–484 (2011).
Armstrong, M. J., Adams, L. A., Canbay, A. & Syn, W.-K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 59, 1174–1197 (2014).
Reiner, Ž . et al. Lysosomal acid lipase deficiency — an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis 235, 21–30 (2014).
Bernstein, D. L., Hülkova, H., Bialer, M. G. & Desnick, R. J. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 58, 1230–1243 (2013).
Ratziu, V., Bellentani, S., Cortez-Pinto, H., Day, C. & Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 53, 372–384 (2010).
Bugianesi, E., McCullough, A. J. & Marchesini, G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 42, 987–1000 (2005).
Alberti, K. G., Zimmet, P., Shaw, J. & IDF Epidemiology Task Force Consensus Group. The metabolic syndrome — a new worldwide definition. Lancet 366, 1059–1062 (2005).
Browning, J. D. Statins and hepatic steatosis: perspectives from the Dallas Heart study. Hepatology 44, 466–471 (2006).
Fraser, A. et al. γ-Glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health study and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 27, 2729–2735 (2007).
Bugianesi, E. et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 39, 179–187 (2004).
Neuschwander-Tetri, B. A. et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 52, 913–924 (2010).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
American Diabetes Association. Standards of medical care in diabetes — 2014. Diabetes Care 37, S14–S80 (2014).
Sasso, M. et al. Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 36, 1825–1835 (2010).
Chan, W.-K., Nik Mustapha, N. R. & Mahadeva, S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 29, 1470–1476 (2014).
Myers, R. P. et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 32, 902–910 (2012).
Karlas, T. et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS ONE 9, e91987 (2014).
Reeder, S. B., Hu, H. H. & Sirlin, C. B. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J. Magn. Reson. Imaging 36, 1011–1014 (2012).
Tang, A. et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 274, 416–425 (2015).
Tang, A. et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267, 422–431 (2013).
Schwimmer, J. B. et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 61, 1887–1895 (2015).
Permutt, Z. et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease — MRI accurately quantifies hepatic steatosis in NAFLD. Aliment. Pharmacol. Ther. 36, 22–29 (2012).
Artz, N. S. et al. Reproducibility of MR-based liver fat quantification across field strength: same-day comparison between 1.5T and 3T in obese subjects. J. Magn. Reson. Imaging 42, 811–817 (2015).
Hines, C. D. G. et al. T1 independent, T2* corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J. Magn. Reson. Imaging 33, 873–881 (2011).
Hansen, K. H., Schroeder, M. E., Hamilton, G., Sirlin, C. B. & Bydder, M. Robustness of fat quantification using chemical shift imaging. Magn. Reson. Imaging 30, 151–157 (2012).
Kleiner, D. E. & Brunt, E. M. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin. Liver Dis. 32, 3–13 (2012).
Marchesini, G. et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37, 917–923 (2003).
European Association for the Study of the Liver. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 63, 237–264 (2015). These are the most recent guidelines for the non-invasive detection of liver fibrosis in liver disease of different aetiology, including NAFLD and NASH.
Fedchuk, L. et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 40, 1209–1222 (2014).
Castera, L., Vilgrain, V. & Angulo, P. Noninvasive evaluation of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 10, 666–675 (2013).
Kwok, R. et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease — the role of transient elastography and plasma cytokeratin-18 fragments. Aliment. Pharmacol. Ther. 39, 254–269 (2014).
Machado, M. V. & Cortez-Pinto, H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J. Hepatol. 58, 1007–1019 (2013).
Awai, H. I., Newton, K. P., Sirlin, C. B., Behling, C. & Schwimmer, J. B. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin. Gastroenterol. Hepatol. 12, 765–773 (2014).
Tang, A., Cloutier, G., Szeverenyi, N. M. & Sirlin, C. B. Ultrasound elastography and MR elastography for assessing liver fibrosis: part 1, principles and techniques. AJR Am. J. Roentgenol. 205, 22–32 (2015).
Sandrin, L. et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29, 1705–1713 (2003).
Bamber, J. et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med. 34, 169–184 (2013).
Ferraioli, G. et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 3: liver. Ultrasound Med. Biol. 41, 1161–1179 (2015).
Myers, R. P. et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology 55, 199–208 (2012).
Bota, S. et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 33, 1138–1147 (2013).
Friedrich-Rust, M. et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur. J. Radiol. 81, e325–e331 (2012).
Muthupillai, R. et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269, 1854–1857 (1995).
Venkatesh, S. K., Yin, M. & Ehman, R. L. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J. Magn. Reson. Imaging 37, 544–555 (2013).
Serai, S. D., Yin, M., Wang, H., Ehman, R. L. & Podberesky, D. J. Cross-vendor validation of liver magnetic resonance elastography. Abdom. Imaging 40, 789–794 (2014).
Kim, D., Kim, W. R., Talwalkar, J. A., Kim, H. J. & Ehman, R. L. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology 268, 411–419 (2013).
Loomba, R. et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 60, 1920–1928 (2014).
Arulanandan, A. et al. Association between quantity of liver fat and cardiovascular risk in patients with nonalcoholic fatty liver disease independent of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 13, 1513–1520 (2015).
Ichikawa, S. et al. Comparison of the diagnostic accuracies of magnetic resonance elastography and transient elastography for hepatic fibrosis. Magn. Reson. Imaging 33, 26–30 (2015).
Neuschwander-Tetri, B. A. Lifestyle modification as the primary treatment of NASH. Clin. Liver Dis. 13, 649–665 (2009).
Johnson, N. A. & George, J. Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology 52, 370–381 (2010).
Vilar-Gomez, E. et al. Weight loss via lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378 (2015). This is the largest trial of lifestyle intervention to date that demonstrated resolution of NASH and improvement or stabilization of fibrosis in those that lost >7% body weight.
Bellentani, S., Dalle Grave, R., Suppini, A. & Marchesini, G. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology 47, 746–754 (2008).
Centis, E., Marzocchi, R., Di Domizio, S., Ciaravella, M. F. & Marchesini, G. The effect of lifestyle changes in non-alcoholic fatty liver disease. Dig. Dis. 28, 267–273 (2010).
Pillai, A. A. & Rinella, M. E. Non-alcoholic fatty liver disease: is bariatric surgery the answer? Clin. Liver Dis. 13, 689–710 (2009).
Lassailly, G. et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 149, 379–388 (2015).
Neuschwander-Tetri, B. A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52, 774–788 (2010).
Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 142, 711–725 (2012).
Fuchs, M. & Sanyal, A. J. Lipotoxicity in NASH. J. Hepatol. 56, 291–293 (2012).
Bouret, S., Levin, B. E. & Ozanne, S. E. Gene–environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol. Rev. 95, 47–82 (2015).
Wisløff, U. et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307, 418–420 (2005).
Cypess, A. M. et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
Crane, J. D. et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 21, 166–172 (2014).
Neuschwander-Tetri, B. A. Food energy efficiency, cannabinoids, and a slow death of the weight loss dogma. Hepatology 46, 12–15 (2007).
Kalaany, N. Y. & Mangelsdorf, D. J. LXRS and FXR: the Yin and Yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 68, 159–191 (2006).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Lomonaco, R. et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55, 1389–1397 (2012).
Gastaldelli, A. et al. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology 50, 1087–1093 (2009).
Sanyal, A. & Chalasani, N. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Lavine, J. E. et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 305, 1659–1668 (2011).
Doycheva, I. & Loomba, R. Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis (NASH): when to use metformin in nonalcoholic fatty liver disease (NAFLD). Adv. Ther. 31, 30–43 (2014).
Cariou, B., Zaïr, Y., Staels, B. & Bruckert, E. Effects of the new dual PPAR α/δ agonist GFT505 on lipid and glucose homeostasis in abdominally obese patients with combined dyslipidemia or impaired glucose metabolism. Diabetes Care 34, 2008–2014 (2011).
Rolo, A. P., Teodoro, J. S. & Palmeira, C. M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 52, 59–69 (2012).
Musso, G. et al. Nitrosative stress predicts the presence and severity of nonalcoholic fatty liver at different stages of the development of insulin resistance and metabolic syndrome: possible role of vitamin A intake. Am. J. Clin. Nutr. 86, 661–671 (2007).
Hoofnagle, J. H. et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 38, 134–143 (2013).
Sanyal, A. J., Abdelmalek, M. F., Suzuki, A., Cummings, O. W. & Chojkier, M. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a Phase 2 trial. Gastroenterology 147, 377–384 (2014).
Cao, S. S. & Kaufman, R. J. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin. Ther. Targets 17, 437–448 (2013).
Ioannou, G. N., Haigh, W. G., Thorning, D. & Savard, C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J. Lipid Res. 54, 1326–1334 (2013).
Bays, H., Cohen, D. E., Chalasani, N., Harrison, S. A. & The National Lipid Association's Statin Safety Task Force. An assessment by the Statin Liver Safety Task Force: 2014 update. J. Clin. Lipidol. 8, S47–S57 (2014).
Eslami, L., Merat, S., Malekzadeh, R., Nasseri-Moghaddam, S. & Aramin, H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst. Rev. 12, CD008623 (2013).
Dongiovanni, P. et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J. Hepatol. 63, 705–712 (2015).
Ratziu, V. et al. Lack of efficacy of an inhibitor of PDE4 in Phase 1 and 2 trials of patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 12, 1724–1730 (2014).
Ratziu, V. et al. A Phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology 55, 419–428 (2012).
Dan, A. A. et al. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 26, 815–820 (2007).
Afendy, A. et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment. Pharmacol. Ther. 30, 469–476 (2009).
Hickman, I. J. et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 53, 413–419 (2004).
David, K. et al. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology 49, 1904–1912 (2009).
Bray, G. A. The missing link — lose weight, live longer. N. Engl. J. Med. 357, 818–820 (2007).
Sjöström, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 357, 741–752 (2007).
Adams, T. D. et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 357, 753–761 (2007).
Sjöström, L. et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 351, 2683–2693 (2004).
Meek, C. L., Lewis, H. B., Reimann, F., Gribble, F. M. & Park, A. J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptideshttp://dx.doi.org/10.1016/j.peptides.2015.08.013 (2015).
Ryan, K. K. et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188 (2014).
Kuipers, F. & Groen, A. K. FXR: the key to benefits in bariatric surgery? Nat. Med. 20, 337–338 (2014).
Birkmeyer, N. J. O. et al. Hospital complication rates with bariatric surgery in Michigan. JAMA 304, 435–442 (2010).
Jan, A., Narwaria, M. & Mahawar, K. K. A. Systematic review of bariatric surgery in patients with liver cirrhosis. Obes. Surg. 25, 1518–1526 (2015).
Yang, J. D. et al. Hepatocellular carcinoma in olmsted county, Minnesota, 1976–2008. Mayo Clin. Proc. 87, 9–16 (2012).
Malik, S. M., Gupte, P. A., de Vera, M. E. & Ahmad, J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 7, 800–806 (2009).
Younossi, Z. M. et al. Association of non-alcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004–2009. Hepatologyhttp://dx.doi.org/10.1002/hep.28123 (2015).
Welzel, T. M. et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am. J. Gastroenterol. 108, 1314–1321 (2013). This paper includes important data from the SEER database demonstrating that, although the risk of HCC in patients with NASH may be lower than other diseases (for example, in hepatitis C virus infection), its high prevalence makes the population attributable risk of HCC in NASH nearly 40%.
Wong, R. J., Cheung, R. & Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 59, 2188–2195 (2014).
Wideroff, L. et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J. Natl Cancer Inst. 89, 1360–1365 (1997).
Yasui, K. et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 9, 428–433 (2011).
Mittal, S. & El-Serag, H. B. Epidemiology of hepatocellular carcinoma: consider the population. J. Clin. Gastroenterol. 47, S2–S6 (2013).
Jiang, W. et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 5, 8096 (2015).
Mehal, W. Z. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat. Rev. Gastroenterol. Hepatol. 10, 637–644 (2013).
Shen, J., Obin, M. S. & Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Aspects Med. 34, 39–58 (2013).
Boursier, J. & Diehl, A. M. Implication of gut microbiota in nonalcoholic fatty liver disease. PLoS Pathog. 11, e1004559 (2015).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Bedossa, P. et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 56, 1751–1759 (2012).
Nobili, V. et al. Retinol-binding protein 4: a promising circulating marker of liver damage in pediatric nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 575–579 (2009).
Younossi, Z. M. et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH). Obes. Surg. 18, 1430–1437 (2008).
Palekar, N. A., Naus, R., Larson, S. P., Ward, J. & Harrison, S. A. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 26, 151–156 (2006).
Harrison, S. A., Oliver, D., Arnold, H. L., Gogia, S. & Neuschwander-Tetri, B. A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 57, 1441–1447 (2008).
Ratziu, V. et al. Liver fibrosis in overweight patients. Gastroenterology 118, 1117–1123 (2000).
Speliotes, E. K. et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart study. Hepatology 51, 1979–1987 (2010).
Hwang, S. et al. The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transpl. 10, 721–725 (2004).
Malik, A., Cheah, P.-L., Hilmi, I. N., Chan, S. P. & Goh, K.-L. Non-alcoholic fatty liver disease in Malaysia: a demographic, anthropometric, metabolic and histological study. J. Dig. Dis. 8, 58–64 (2007).
Hamaguchi, M. et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 143, 722–728 (2005).
Fan, J.-G. et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J. Hepatol. 43, 508–514 (2005).
Das, K. et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology 51, 1593–1602 (2010).
Kwon, Y.-M. et al. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am. J. Gastroenterol. 107, 1852–1858 (2012).
el-Hassan, A. Y., Ibrahim, E. M., al-Mulhim, F. A., Nabhan, A. A. & Chammas, M. Y. Fatty infiltration of the liver: analysis of prevalence, radiological and clinical features and influence on patient management. Br. J. Radiol. 65, 774–778 (1992).
Aboueisha, H. et al. A retrospective evaluation of causes of exempting living liver donors in an Egyptian centre. Arab J. Gastroenterol. 14, 10–13 (2013).
Strauss, R. S., Barlow, S. E. & Dietz, W. H. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J. Pediatr. 136, 727–733 (2000).
Zou, C. C., Liang, L., Hong, F., Fu, J. F. & Zhao, Z. Y. Serum adiponectin, resistin levels and non-alcoholic fatty liver disease in obese children. Endocr. J. 52, 519–524 (2005).
Guzzaloni, G., Grugni, G., Minocci, A., Moro, D. & Morabito, F. Liver steatosis in juvenile obesity: correlations with lipid profile, hepatic biochemical parameters and glycemic and insulinemic responses to an oral glucose tolerance test. Int. J. Obes. Relat. Metab. Disord. 24, 772–776 (2000).
Fu, J. et al. Nonalcoholic steatohepatitis in obese children: the prevalence and possible mechanism. Zhejiang Da Xue Xue Bao Yi Xue Ban 35, 64–68 (in Chinese) (2006).
Schwimmer, J. B. et al. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J. Pediatr. 143, 500–505 (2003).
Nobili, V. et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology 44, 458–465 (2006).
Feldstein, A. E. et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut 58, 1538–1544 (2009).
Bedogni, G. et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006). The Fatty Liver Index is the first and most widely used index for the non-ultrasound detection of NAFLD.
Kotronen, A. et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 137, 865–872 (2009).
Poynard, T. et al. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS ONE 7, e30325 (2012).
Angulo, P. et al. The NAFLD Fibrosis Score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854 (2007). The NAFLD Fibrosis Score is the only non-invasive index for the identification of NASH that has been validated both cross-sectionally and longitudinally.
Ratziu, V. et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 6, 6 (2006).
Shah, A. G. et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1104–1112 (2009).
Adams, L. A. et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 26, 1536–1543 (2011).
Guha, I. N. et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 47, 455–460 (2008).
Acknowledgements
E.B. is a member of the EPoS (Elucidating Pathways of Steatohepatitis) consortium funded by the Horizon 2020 Framework Programme of the European Union under Grant Agreement 634413.
Author information
Authors and Affiliations
Contributions
Introduction (E.M.B.); Epidemiology (V.W.-S.W., V.N. and E.M.B.); Mechanisms/pathophysiology (C.P.D., S.S. and J.J.M.); Diagnosis, screening and prevention (E.B. and C.B.S.); Management (B.A.N.-T.); Quality of life (M.E.R.); Outlook (M.E.R.); Overview of Primer (E.M.B.).
Corresponding author
Ethics declarations
Competing interests
E.M.B. has received consulting fees from Eli Lilly and acted as a paid study pathologist for Rottapharm. C.B.S. has received research grants from GE Healthcare and Siemens. V.W.-S.W. is or has been an advisory board member for Gilead and Janssen, a consultant for AbbVie, Merck and NovoMedica, and has received lecture fees from AbbVie, Echosens and Gilead. B.A.N.-T. has received consulting fees from Nimbus Therapeutics, Bristol-Myers Squibb, Janssen, Conatus, Scholar Rock, Novartis, Galmed, Zafgen and Pfizer. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Brunt, E., Wong, VS., Nobili, V. et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers 1, 15080 (2015). https://doi.org/10.1038/nrdp.2015.80
Published:
DOI: https://doi.org/10.1038/nrdp.2015.80
This article is cited by
-
Dynamic changes in the mouse hepatic lipidome following warm ischemia reperfusion injury
Scientific Reports (2024)
-
A high-trans fat, high-carbohydrate, high-cholesterol, high-cholate diet-induced nonalcoholic steatohepatitis mouse model and its hepatic immune response
Nutrition & Metabolism (2023)
-
High-fat diet in early life triggers both reversible and persistent epigenetic changes in the medaka fish (Oryzias latipes)
BMC Genomics (2023)
-
Relevance and consequence of chronic inflammation for obesity development
Molecular and Cellular Pediatrics (2023)
-
Weigh change across adulthood is related to the presence of NAFLD: results from NHANES III
Journal of Translational Medicine (2023)