Key Points
-
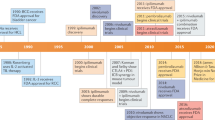
The concept of immuno-oncology, using the immune system to fight cancer, dates back 150 years.
-
However, broad clinical success for immunotherapies in cancer has only recently been achieved and comprises a class of biologics that includes vaccines, engineered immune cells and mAbs.
-
Some areas of immune biology cannot be modulated with biologic therapies either due to intracellular access restriction or enzymatic properties that require smaller moieties for intervention.
-
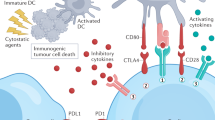
Most small-molecule drugs are tumour-targeted agents, some of which induce immunogenic cell death that could assist an immune response or create synergy in combination with an immunotherapy.
-
Multiple small-molecule drugs aiming to block the function of immune suppressor cells (for example, myeloid-derived suppressor cells, regulatory T cells, dendritic cells and tumour-associated macrophages) have been identified.
-
The hypoxic environment of solid tumours creates a hypoxia–adenosinergic axis of gene regulation, which enforces tumour immune tolerance and can be targeted by small-molecule drugs.
-
Small-molecule immunotherapy for cancer is a growing area ripe with opportunity for exploitation of critical immune biology and potential new drugs to offer patient benefit.
Abstract
The regulatory approval of ipilimumab (Yervoy) in 2011 ushered in a new era of cancer immunotherapies with durable clinical effects. Most of these breakthrough medicines are monoclonal antibodies that block protein–protein interactions between T cell checkpoint receptors and their cognate ligands. In addition, genetically engineered autologous T cell therapies have also recently demonstrated significant clinical responses in haematological cancers. Conspicuously missing from this class of therapies are traditional small-molecule drugs, which have previously served as the backbone of targeted cancer therapies. Modulating the immune system through a small-molecule approach offers several unique advantages that are complementary to, and potentially synergistic with, biologic modalities. This Review highlights immuno-oncology pathways and mechanisms that can be best or solely targeted by small-molecule medicines. Agents aimed at these mechanisms — modulation of the immune response, trafficking to the tumour microenvironment and cellular infiltration — are poised to significantly extend the scope of immuno-oncology applications and enhance the opportunities for combination with tumour-targeted agents and biologic immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Coley, W. B. Contribution to the knowledge of sarcoma. Ann. Surg. 14, 199–220 (1891).
Cheever, M. A. & Higano, C. S. Provenge (sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 17, 3520–3526 (2011).
Hoos, A. et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin. Oncol. 37, 533–546 (2010). Recounts lessons learnt from the development of ipilimumab (a CTLA4-specific mAb), the first modern immuno-oncology therapy for melanoma.
Hoos, A., Britten, C. M., Huber, C. & O'Donnell-Tormey, J. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat. Biotech. 29, 867–870 (2011).
Poole, R. M. Pembrolizumab: first global approval. Drugs 74, 1973–1981 (2014). Summarizes the milestones in the development of pembrolizumab (a PD1-specific mAb) for the treatment of malignant melanoma.
Wang, C. et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2, 846–856 (2014).
Lee, S. & Margolin, K. Cytokines in cancer immunotherapy. Cancers 3, 3856–3893 (2011).
Melero, I. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524 (2014).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Muller, A. J. & Scherle, P. A. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat. Rev. Cancer 6, 613–625 (2006). The first comprehensive review of small-molecule drug targets with the potential to treat cancer by relieving immune tolerance.
Gill, S. Going viral: chimeric antigen receptor T cell therapy for hematological malignancies. Immunol. Rev. 263, 68–89 (2015).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
Marabelle, A. et al. Depleting tumor-specific T regs at a single site eradicates disseminated tumors. J. Clin. Invest. 123, 2447–2463 (2013).
Coulie, P. G., Van den Eynde, B. J., van der Bruggen, P. & Boon, T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014).
Srinivasan, R., Houghton, A. N. & Wolchok, J. D. Induction of autoantibodies against tyrosinase-related proteins following DNA vaccination: unexpected reactivity to a protein paralogue. Cancer Immun. 2, 8 (2002).
Printz, C. Spontaneous regression of melanoma may offer insight into cancer immunology. J. Natl Cancer Inst. 93, 1047–1048 (2001).
Waldhauer, I. & Steinle, A. NK cells and cancer immunosurveillance. Oncogene 27, 5932–5943 (2008).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Sica, A. & Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 117, 1155–1166 (2007).
Lewis, C. & Murdoch, C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am. J. Pathol. 167, 627–635 (2005).
Almand, B. et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689 (2001).
Frey, D. M. et al. High frequency of tumor-infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int. J. Cancer 126, 2635–2643 (2010).
Diaz-Montero, C. M. et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 (2009).
Whiteside, T. L. Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin. Biol. Ther. 14, 1411–1425 (2014).
Adeegbe, D. O. & Nishikawa, H. Natural and induced T regulatory cells in cancer. Front. Immunol. 4, 190 (2013).
Madar, S. Goldstein, I. & Rotter, V. 'Cancer associated fibroblasts' — more than meets the eye. Trends Mol. Med. 19, 447–453 (2013).
Galluzzi, L., Senovilla, L., Zitvogel, L. & Kroemer, G. The secret ally: immunostimulation by anticancer drugs. Nat. Rev. Drug Discov. 11, 215–233 (2012).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013). A comprehensive review that outlines the characteristics and consequences of immunogenic cell death in cancer therapy.
Sullivan, R. J., LoRusso, P. M. & Flaherty, K. T. The intersection of immune-directed and molecularly targeted therapy in advanced melanoma: where we have been, are, and will be. Clin. Cancer Res. 19, 5283–5291 (2013).
Lynch, T. J. et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non–small-cell lung cancer: results from a randomized, double-blind, multicenter Phase II study. J. Clin. Oncol. 30, 2046–2054 (2012).
Ribas, A., Hodi, F. S., Callahan, M., Konto, C. & Wolchok, J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 368, 1365–1366 (2013).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Liu, X. et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 115, 3520–3530 (2010). Provides an in vitro and in vivo demonstration that a potent and selective IDO1 inhibitor can effectively regulate immune tolerance and slow tumour growth.
Friberg, M. et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer 101, 151–155 (2002).
Mautino, M. R., et al. Synergistic antitumor effects of combinatorial immune checkpoint inhibition with anti-PD-1/PD-L antibodies and the IDO pathway inhibitors NLG-919 and indoximod in the context of active immunotherapy. [abstract]. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5–9; San Diego, CA. Philadelphia (PA): AACR. Cancer Res. 74 (Suppl. 19), 5023 (2014).
Pilotte, L. et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl Acad. Sci. USA 109, 2497–2502 (2012).
Van Zandt, M. C. et al. Discovery of (R)-2-amino-6-borono-2-(2-(piperidin-1-yl)ethyl)hexanoic acid and congeners as highly potent inhibitors of human arginases I and II for treatment of myocardial reperfusion injury. J. Med. Chem. 56, 2568–2580 (2013).
Hagos, G. K. et al. Anti-inflammatory, antiproliferative, and cytoprotective activity of NO chimera nitrates of use in cancer chemoprevention. Mol. Pharmacol. 74, 1381–1391 (2008).
Molon, B. et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 208, 1949–1962 (2011).
Serafini, P. et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 203, 2691–2702 (2006).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009). Elucidates the mechanism for the effect of high extracellular ATP acting on dendritic cells leading to IL-1β release and enhanced tumour-specific CD8 T cell cytotoxicity.
Adinolfi, E. et al. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 72, 2957–2969 (2012).
Meis, S. et al. NF546 [4,4′-(carbonylbis(imino-3,1- phenylene-carbonylimino-3,1-(4-methyl-phenylene)-carbonylimino))-bis(1,3-xylene-alpha,alpha′-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J. Pharmacol. Exp. Ther. 332, 238–247 (2010).
Beavis, P. A. et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc. Natl Acad. Sci. USA 110, 14711–14716 (2013).
Chen, J. F., Eltzschig, H. K. & Fredholm, B. B. Adenosine receptors as drug targets — what are the challenges? Nat. Rev. Drug Discov. 12, 265–286 (2013).
Iannone, R., Miele, L., Maiolino, P., Pinto, A. & Morello, S. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia 15, 1400–1409 (2013).
Bastid, J. et al. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 32, 1743–1751 (2013).
Wang, L. et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J. Clin. Invest. 121, 2371–2382 (2011).
Wang, D. & DuBois, R. N. The role of anti-inflammatory drugs in colorectal cancer. Ann. Rev. Med. 64, 131–144 (2013).
af Forselles, K. J. et al. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP 2 receptor antagonist. Br. J. Pharmacol. 164, 1847–1856 (2011).
Ma, X. et al. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology 2, e22647 (2013).
Highfill, S. L. et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 6, 237ra67 (2014).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Sanford, D. E. et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 19, 3404–3415 (2013).
Weitzenfeld, P. & Ben-Baruch, A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett. 352, 36–53 (2014).
Onier, N. et al. Cure of colon cancer metastasis in rats with the new lipid A OM 174. Apoptosis of tumor cells and immunization of rats. Clin. Exp. Metastasis 17, 299–306 (1999).
Geisse, J. et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 50, 722–733 (2004).
Dudek, A. Z. et al. First in human phase I trial of 852A, a novel systemic Toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clin. Cancer Res. 13, 7119–7125 (2007).
Northfelt, D. W. et al. A phase I dose-finding study of the novel toll-like receptor 8 agonist VTX-2337 in adult subjects with advanced solid tumors or lymphoma. Clin. Cancer Res. 20, 3683–3691 (2014).
Hwang, J. J. et al. A phase I study of HYB2055 in patients (pts) with advanced solid malignancies. J. Clin. Oncol. 22 (Suppl.), 3111 (2004).
Yoon, J. H. et al. Activin receptor-like kinase5 inhibition suppresses mouse melanoma by ubiquitin degradation of Smad4, thereby derepressing eomesodermin in cytotoxic T lymphocytes. EMBO Mol. Med. 5, 1720–1739 (2013).
Khalili, J. S. et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin. Cancer Res. 18, 5329–5340 (2012).
Schilling, B. et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int. J. Cancer 133, 1653–1663 (2013).
Eyob, H., Ekiz, H. A. & Welm, A. L. RON promotes the metastatic spread of breast carcinomas by subverting antitumor immune responses. Oncoimmunology 2, e25670 (2013).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Ali, K. et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 510, 407–411 (2014). Demonstrates that a PI3Kδ-selective inhibitor can break tumour-induced immune tolerance in a wide range of solid tumours.
Schmid, Michael, C. et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell 19, 715–727 (2011).
Sikalidis, A. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol. Oncol. Res. 21, 9–17 (2015).
Mellor, A. L. & Munn, D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4, 762–774 (2004). A seminal review detailing the involvement of IDO1 in the development of immune tolerance.
Okamoto, A. et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin. Cancer Res. 11, 6030–6039 (2005).
Belladonna, M. L., Orabona, C., Grohmann, U. & Puccetti, P. TGF-β and kynurenines as the key to infectious tolerance. Trends Mol. Med. 15, 41–49 (2009).
Opitz, C. A. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011).
Yue, E. W. et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J. Med. Chem. 52, 7364–7367 (2009).
Fallarino, F. et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 176, 6752–6761 (2006).
Rodriguez, P. C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Rodriguez, P. C. et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69, 1553–1560 (2009).
de Boniface, J., Mao, Y., Schmidt-Mende, J., Kiessling, R. & Poschke, I. Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology 1, 1305–1312 (2012).
Serafini, P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol. Res. 57, 172–184 (2013).
De Santo, C. et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl Acad. Sci. USA 102, 4185–4190 (2005).
Gallina, G. et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 116, 2777–2790 (2006).
Califano, J. A. et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 30–38 (2015).
Weed, D. T. et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 39–48 (2015). Provides clinical evidence in patients with HNSCC that a PDE5 inhibitor can lower the numbers of MDSCs and T Reg cells and increase the number of tumour-specific CD8+ T cells.
Sitkovsky, M. & Ohta, A. Targeting the hypoxia-adenosinergic signaling pathway to improve the adoptive immunotherapy of cancer. J. Mol. Med. 91, 147–155 (2013).
Blay, J., White, T. D. & Hoskin, D. W. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 57, 2602–2605 (1997).
Yang, M. et al. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol. Cell Biol. 88, 165–171 (2010).
Sitkovsky, M. V. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 30, 102–108 (2009). Hypothesis detailing how the hypoxia–adenosinergic axis enforces tumour immune tolerance through the regulation of T Reg cell function.
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007). Demonstrates the regulation of T Reg cell–effector T cell interaction by coordinate expression of CD39, CD73 and A 2A receptors.
Ohta, A. et al. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 3, 190 (2012).
Sun, X. et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 139, 1030–1040 (2010).
Chalmin, F. et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36, 362–373 (2012).
Aswad, F., Kawamura, H. & Dennert, G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J. Immunol. 175, 3075–3083 (2005).
Schenk, U. et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 4, ra12 (2011).
Bian, S. et al. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS ONE 8, e60184 (2013).
Di Virgilio, F., Ferrari, D. & Adinolfi, E. P2X7: a growth-promoting receptor — implications for cancer. Purinergic Signal. 5, 251–256 (2009).
Bianchi, G. et al. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis. 5, e1135 (2014).
North, R. A. & Jarvis, M. F. P2X receptors as drug targets. Mol. Pharmacol. 83, 759–769 (2013).
Csóka, B. et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 26, 376–386 (2012).
Ryzhov, S. et al. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J. Immunol. 187, 6120–6129 (2011).
Cekic, C. et al. Adenosine A 2B receptor blockade slows growth of bladder and breast tumors. J. Immunol. 188, 198–205 (2012).
Kalla, R. V. & Zablocki, J. Progress in the discovery of selective, high affinity A2B adenosine receptor antagonists as clinical candidates. Purinergic Signal. 5, 21–29 (2009).
Borsellino, G. et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110, 1225–1232 (2007).
Jie, H. B. et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer 109, 2629–2635 (2013).
Serra, S. et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood 118, 6141–6152 (2011).
Hilchey, S. P. et al. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J. Immunol. 183, 6157–6166 (2009).
Michaud, M. et al. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology 1, 393–395 (2012).
Al-Rashida, M. & Iqbal, J. Therapeutic potentials of ecto-nucleoside triphosphate diphosphohydrolase, ecto-nucleotide pyrophosphatase/phosphodiesterase, ecto-5′-nucleotidase, and alkaline phosphatase inhibitors. Med. Res. Rev. 34, 703–743 (2014).
Lévesque, S. A., Lavoie, É. G., Lecka, J., Bigonnesse, F. & Sévigny, J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br. J. Pharmacol. 152, 141–150 (2007).
Lecka, J. et al. 8-BuS-ATP derivatives as specific NTPDase1 inhibitors. Br. J. Pharmacol. 169, 179–196 (2013).
Synnestvedt, K. et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Stagg, J. & Smyth, M. J. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29, 5346–5358 (2010).
Mandapathil, M. et al. Adenosine and prostaglandin e2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J. Biol. Chem. 285, 27571–27580 (2010).
Clayton, A., Al-Taei, S., Webber, J., Mason, M. D. & Tabi, Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 187, 676–683 (2011).
Schuler, P. J. et al. Human CD4+CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin. Exp. Immunol. 177, 531–543 (2014).
Stagg, J. et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl Acad. Sci. USA 107, 1547–1552 (2010).
Xu, S. et al. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro. Oncol. 15, 1160–1172 (2013).
Forte, G. et al. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J. Immunol. 189, 2226–2233 (2012).
Knapp, K. et al. Crystal structure of the human ecto-5′-nucleotidase (CD73): insights into the regulation of purinergic signaling. Structure 20, 2161–2173 (2012).
Ogino, S. et al. Cyclooxygenase-2 expression Is an independent predictor of poor prognosis in colon cancer. Clin. Cancer Res. 14, 8221–8227 (2008).
Kaidi, A., Qualtrough, D., Williams, A. C. & Paraskeva, C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 66, 6683–6691 (2006).
Nakanishi, Y. et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis 32, 1333–1339 (2011).
Mahic, M., Yaqub, S., Johansson, C. C., Taskén, K. & Aandahl, E. M. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J. Immunol. 177, 246–254 (2006).
Obermajer, N., Muthuswamy, R., Lesnock, J., Edwards, R. P. & Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118, 5498–5505 (2011).
Duan, B. et al. Distinct roles of adenylyl cyclase VII in regulating the immune responses in mice. J. Immunol. 185, 335–344 (2010).
Mao, Y. et al. Inhibition of tumor-derived prostaglandin-E2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 20, 4096–4106 (2014).
Holt, D., Ma, X., Kundu, N. & Fulton, A. Prostaglandin E2 (PGE2) suppresses natural killer cell function primarily through the PGE2 receptor EP4. Cancer Immunol. Immunother. 60, 1577–1586 (2011).
Antonova, M. et al. The pharmacological effect of BGC20-1531, a novel prostanoid EP 4 receptor antagonist, in the prostaglandin E 2 human model of headache. J. Headache Pain 12, 551–559 (2011).
Jiang, L. I., Collins, J., Davis, R., Fraser, I. D. & Sternweis, P. C. Regulation of cAMP responses by the G12-13 pathway converges on adenylyl cyclase VII. J. Biol. Chem. 283, 23429–23439 (2008).
Whiteside, T. L. & Jackson, E. K. Adenosine and prostaglandin E2 production by human inducible regulatory T cells in health and disease. Front. Immunol. 4, 212 (2013). Inhibitors of adenylyl cyclase VII are proposed as a novel strategy to disarm the immunosuppressive function of T Reg cells.
Mancini, R. J., Stutts, L., Ryu, K. A., Tom, J. K. & Esser-Kahn, A. P. Directing the immune system with chemical compounds. ACS Chem. Biol. 9, 1075–1085 (2014).
Pradere, J. P., Dapito, D. H. & Schwabe, R. F. The yin and yang of Toll-like receptors in cancer. Oncogene 33, 3485–3495 (2014).
Guiducci, C., Vicari, A. P., Sangaletti, S., Trinchieri, G. & Colombo, M. P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 65, 3437–3446 (2005).
Fang, H. et al. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell. Mol. Immunol. 11, 150–159 (2014).
Peri, F. & Calabrese, V. Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: an update. J. Med. Chem. 57, 3612–3622 (2014).
Gérard, C., Baudson, N., Ory, T. & Louahed, J. Tumor mouse model confirms MAGE-A3 cancer immunotherapeutic as an efficient inducer of long-lasting anti-tumoral responses. PLoS ONE 9, e94883 (2014).
Kaczanowska, S., Joseph, A. M. & Davila, E. TLR agonists: our best frenemy in cancer immunotherapy. J. Leukocyte Biol. 93, 847–863 (2013). A review integrating recent advances in TLR signalling with the thus far disappointing clinical results to develop criteria for future clinical development of immune-stimulant TLR agonists for cancer treatment.
Zlotnik, A. & Yoshie, O. The chemokine superfamily revisited. Immunity 36, 705–712 (2012).
Franciszkiewicz, K., Boissonnas, A., Boutet, M., Combadière, C. & Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 72, 6325–6332 (2012).
Vinader, V. & Afarinkia, K. A beginners guide to chemokines. Future Med. Chem. 4, 845–852 (2012).
Stewart, T. J. & Smyth, M. J. Chemokine–chemokine receptors in cancer immunotherapy. Immunotherapy 1, 109–127 (2009).
Vinader, V. & Afarinkia, K. The emerging role of CXC chemokines and their receptors in cancer. Future Med. Chem. 4, 853–867 (2012).
Debnath, B., Xu, S., Grande, F., Garofalo, A. & Neamati, N. Small molecule inhibitors of CXCR4. Theranostics 3, 47–75 (2013).
Yan, M. et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 13, R47 (2011).
Varmavuo, V., Mäntymaa, P., Kuittinen, T., Nousiainen, T. & Jantunen, E. Pre-emptive plerixafor injection increases blood neutrophil, lymphocyte and monocyte counts in addition to CD34+ counts in patients with non-Hodgkin lymphoma mobilizing poorly with chemotherapy plus G-CSF: potential implications for apheresis and graft composition. Transfusion Apheresis Sci. 46, 257–262 (2012).
Peled, A. et al. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin. Cancer Res. 20, 469–479 (2014).
Katoh, H. et al. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 24, 631–644 (2013).
Stewart, T. J. & Smyth, M. J. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 30, 125–140 (2011).
Anderton, M. J. et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol. Pathol. 39, 916–924 (2011).
Rodon, J. et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res. 21, 553–600 (2015).
Jin, C. H. et al. Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin- 2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-β type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J. Med. Chem. 57, 4213–4238 (2014).
Moran, N. Incyte comes of age with JAK inhibitor approval. Nat. Biotech. 30, 3–5 (2012).
Furgan, M. et al. STAT inhibitors for cancer therapy. J. Hematol. Oncol. 6, 90 (2013).
DeNardo, D. G. et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67 (2011).
Balachandran, V. P. et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 17, 1094–1100 (2011).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Liu, L. et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD1, PD-L1 and CTLA-4. Clin. Cancer Res. 21, 1639–1651 (2015). Studies in human T cells using BRAF and MEK inhibitors in combination with checkpoint blockers provide a translational rationale for clinical trials to further extend their utility.
Yao, H. P., Zhou, Y. Q., Zhang, R. & Wang, M. H. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat. Rev. Cancer 13, 466–481 (2013).
Burger, J. A. & Okkenhaug, K. Haematological cancer: Idelalisib-targeting PI3Kδ in patients with B-cell malignancies. Nat. Rev. Clin. Oncol. 11, 184–186 (2014).
Smyth, M. J., Ngiow, S. F. & Teng, M. W. L. Targeting regulatory T cells in tumor immunotherapy. Immunol. Cell Biol. 92, 473–474 (2014).
Stewart, C. A. et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J. Clin. Invest. 123, 4859–4874 (2013).
Acknowledgements
This Review was inspired by the bravery and resolve of cancer patients and their families. The authors also wish to thank R&D leadership as well as immuno-oncology researchers, physicians and administrative staff at GlaxoSmithKline for their continued devotion to cancer medicine innovation, with particular thanks to A. Lockenour for thoughtful discussion and review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.L.A., J.S., R.S. and A.H. are employees of GlaxoSmithKline. J.L.A., J.S. and R.S. also hold GlaxoSmithKline stocks.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Combination Immunotherapy: Small Molecules that Boost Immune Response in Combination with Other Agents (PDF 426 kb)
Supplementary information S2 (table)
Immuno–Modulating SMDs that have Entered Clinical Trials Either as Single–Agent Studies or as Part of a Combination Therapy Exploration. (PDF 741 kb)
Glossary
- Dendritic cells
-
Professional antigen-presenting cells that take up and present antigens. In the tumour microenvironment these cells present antigens from dying tumour cells, which are taken up and processed by immature dendritic cells. Upon cell maturation, and as they migrate to the draining lymph node, they display antigen via HLA class I and II molecules to prime effector T lymphocytes.
- Effector T cell
-
Mediates killing of target cells via cognate antigen recognition on HLA class I molecules.
- Regulatory (TReg) cell
-
CD4+CD25+FOXP3+ cells that are suppressive in nature and dampen CD8+ effector T cell responses.
- Natural killer cells
-
Cells that kill viral and tumour targets via non-MHC restriction.
- Tumour-associated macrophages
-
(TAMs). Arise from anti-inflammatory, pro-tumorigenic M2 macrophages and reside in the tumour stroma, causing inhibition of immune responses, or in blood vessels in the core of the tumour tissue, promoting tumour invasion.
- TH1-type response
-
A CD4+ T cell immune response mediated by pro-inflammatory cytokines (e.g., interferon, interleukin-1 and tumour necrosis factor), which promotes cellular immune responses.
- TH2-type response
-
A CD4+ T cell response driven by interleukin-4, which stimulates antibody production.
- Myeloid-derived suppressor cells
-
(MDSCs). Cells that promote immune suppression of multiple cell types and initiate tumour cell evasion.
- Cancer-associated fibroblasts
-
Components of tumour tissue that support and promote tumour growth and immune cell evasion.
- Immunogenic cell death
-
Cell death that primes an immune response; characterized by release of ATP, high mobility group box 1 (HMGB1) and the pre-apoptotic display of calreticulin.
- CD39
-
Also known as ectonucleoside triphosphate diphosphohydrolase 1 (NTPDase 1), CD39 is a cell surface-bound phosphatase found on lymphocytes and tumours and is responsible for the conversion of extracellular ATP into adenosine.
- CD73
-
Also known as 5′-nucleotidase (5′-NT), CD73 is a cell surface-bound phosphatase found on lymphocytes and tumours and is responsible for the conversion of extracellular ATP into adenosine.
- Hypoxia–adenosinergic axis
-
A programme of gene regulation in response to hypoxia whereby elevated extracellular adenosine mediates a broadly suppressive immune response.
- B cells
-
Lymphocytes that produce antibodies in response to antigen presentation by antigen-presenting cells. B cells may also function as antigen-presenting cells in some instances.
Rights and permissions
About this article
Cite this article
Adams, J., Smothers, J., Srinivasan, R. et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov 14, 603–622 (2015). https://doi.org/10.1038/nrd4596
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4596
This article is cited by
-
Chelator impact: investigating the pharmacokinetic behavior of copper-64 labeled PD-L1 radioligands
EJNMMI Radiopharmacy and Chemistry (2024)
-
A promising target for breast cancer: B7-H3
BMC Cancer (2024)
-
High expression of CDKN2A is associated with poor prognosis in colorectal cancer and may guide PD-1-mediated immunotherapy
BMC Cancer (2023)
-
Levels of systemic inflammation response index are correlated with tumor-associated bacteria in colorectal cancer
Cell Death & Disease (2023)
-
Leveraging structural and 2D-QSAR to investigate the role of functional group substitutions, conserved surface residues and desolvation in triggering the small molecule-induced dimerization of hPD-L1
BMC Chemistry (2022)