Key Points
-
The first goal of the current article is to give an overview of the biochemistry and the pathophysiological actions of peroxynitrite (ONOO−), a short-lived oxidant species formed by the diffusion-controlled reaction of nitric oxide (•NO) with a superoxide radical (O2•−). The second goal of the article is to outline the therapeutic implications of peroxynitrite, and to give an overview of the various pharmacological classes of peroxynitrite scavengers and peroxynitrite decomposition catalysts.
-
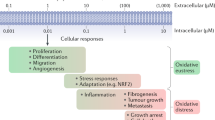
Peroxynitrite induces cell death, and can influence signal-transduction processes, mitochondrial function and signalling of apoptosis. The formation and reactions of peroxynitrite play a significant role in various diseases. Products of peroxynitrite reactions with macromolecules have been detected in several pathophysiological conditions, including vascular diseases, ischaemia–reperfusion injury, circulatory shock, inflammation, pain and neurodegeneration. In these conditions, pharmacological inhibition of the formation or action of peroxynitrite was shown to be of benefit.
-
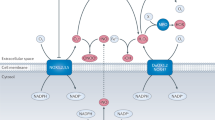
The biological chemistry of peroxynitrite is highly pH-dependent and is dictated primarily by reactions with thiols, carbon dioxide and transition-metal centres. Reaction of peroxynitrite and/or peroxynitrite-derived radicals (for example, carbonate and nitrogen dioxide radicals) with targets results in one- and two-electron oxidations and nitration. Diffusion of peroxynitrite through biomembranes can cause oxidative damage at one to two cell diameters from its site of formation.
-
The most advanced pharmacological strategies to attenuate the toxic effects of peroxynitrite involve its fast (k>1 × 106 M−1s−1) catalytic reduction to nitrite (NO2) or its isomerization to nitrate (NO3) by metalloporphyrins. Manganese and iron metalloporphyrinic compounds have been shown to rapidly react with peroxynitrite and promote its decomposition in a catalytic fashion. Such compounds — including manganese (III) meso-tetrakis((N-ethyl) pyridynium-2-yl) l porphyrin (MnTE-2-PyP), manganese (III) tetrakis(N-N′-diethylimidazolium-2-yl)porphyrin (AEOL-10150) and FeCl tetrakis-2-(triethylene glycol monomethyl ether) pyridyl porphyrin (FP15) — attenuate peroxynitrite-dependent toxicity in vitro and in vivo, and emerge as candidates for drug development for the therapy of cardiovascular, inflammatory and neurodegenerative diseases.
Abstract
Peroxynitrite — the product of the diffusion-controlled reaction of nitric oxide with superoxide radical — is a short-lived oxidant species that is a potent inducer of cell death. Conditions in which the reaction products of peroxynitrite have been detected and in which pharmacological inhibition of its formation or its decomposition have been shown to be of benefit include vascular diseases, ischaemia–reperfusion injury, circulatory shock, inflammation, pain and neurodegeneration. In this Review, we first discuss the biochemistry and pathophysiology of peroxynitrite and then focus on pharmacological strategies to attenuate the toxic effects of peroxynitrite. These include its catalytic reduction to nitrite and its isomerization to nitrate by metalloporphyrins, which have led to potential candidates for drug development for cardiovascular, inflammatory and neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beckman, J. S. et al. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from NO and superoxide. Proc. Natl Acad. Sci. USA 87, 1620–1624 (1990). The first report implicating the biological formation and pathophysiological potential of peroxynitrite.
Gryglewski, R. J., Palmer, R. M. & Moncada, S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320, 454–456 (1986).
Radi, R. et al. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 30, 463–488 (2001).
Alvarez, M. N., Piacenza, L., Irigoin, F., Peluffo, G. & Radi, R. Macrophage-derived peroxynitrite diffusion and toxicity to Trypanosoma cruzi. Arch. Biochem. Biophys. 432, 222–232 (2004).
Nalwaya, N. & Deen, W. M. NO, oxygen, and superoxide formation and consumption in macrophage cultures. Chem. Res. Toxicol. 18, 486–493 (2005).
Quijano, C., Romero, N. & Radi, R. Tyrosine nitration by superoxide and NO fluxes in biological systems: modeling the impact of superoxide dismutase and NO diffusion. Free Radic. Biol. Med. 39, 728–741 (2005).
Marla, S. S., Lee, J. & Groves, J. T. Peroxynitrite rapidly permeates phospholipid membranes. Proc. Natl Acad. Sci. USA 94, 14243–14248 (1997).
Denicola, A., Souza, J. M. & Radi, R. Diffusion of peroxynitrite across erythrocyte membranes. Proc. Natl Acad. Sci. USA 95, 3566–3571 (1998).
Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. Peroxynitrite oxidation of sulfhydryls: the cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266, 4244–4250 (1991). The first demonstration that peroxynitrite can oxidize sulphydryls, and the application of stopped-flow spectrophotometry for rate constant determination.
Bartesaghi, S. et al. Mechanistic studies of peroxynitrite-mediated tyrosine nitration in membranes using the hydrophobic probe N-t-BOC-L-tyrosine tert-butyl ester. Biochemistry 45, 6813–6825 (2006).
Quijano, C., Alvarez, B., Gatti, R., Augusto, O. & Radi, R. Pathways of peroxynitrite oxidation of thiol groups. Biochem. J. 322, 167–173 (1997).
Carballal, S. et al. Sulfenic acid formation in human serum albumin. Biochemistry 42, 9906–9914 (2003).
Lymar, S. V. & Hurst, J. K. Radical nature of peroxynitrite reactivity. Chem. Res. Toxicol. 11, 714–715 (1998).
Goldstein, S., Lind, J. & Merenyi, G. Chemistry of peroxynitrites as compared to peroxynitrates. Chem. Rev. 105, 2457–2470 (2005).
Romero, N. et al. Reaction of human hemoglobin with peroxynitrite: isomerization to nitrate and secondary formation of protein radicals. J. Biol. Chem. 278, 44049–44057 (2003).
Radi, R. NO, oxidants, and protein tyrosine nitration. Proc. Natl Acad. Sci. USA 101, 4003–4008 (2004).
Bonini, M. G. & Augusto, O. Carbon dioxide stimulates the production of thiyl, sulfinyl, and disulfide radical anion from thiol oxidation by peroxynitrite. J. Biol. Chem. 276, 9749–54 (2001).
Salgo, M. G., Bermudez, E., Squadrito, G. L. & Pryor, W. A. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes. Arch. Biochem. Biophys. 322, 500–505 (1995).
Szabó, C. & Ohshima, H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1, 373–385 (1997).
Burney, S., Niles, J. C., Dedon, P. C. & Tannenbaum, S. R. DNA damage in deoxynucleosides and oligonucleotides treated with peroxynitrite. Chem. Res. Toxicol. 12, 513–520 (1999).
Niles, J. C., Wishnok, J. S. & Tannenbaum, S. R. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide 14, 109–121 (2006).
Kennedy, L. J., Moore, K. Jr, Caulfield, J. L., Tannenbaum, S. R. & Dedon, P. C. Quantitation of 8-oxoguanine and strand breaks produced by four oxidizing agents. Chem. Res. Toxicol. 10, 386–392 (1997).
Villa, L. M., Salas, E., Darley-Usmar, V. M., Radomski, M. W. & Moncada, S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc. Natl Acad. Sci. USA 91, 12383–12387 (1994).
Rubbo, H. et al. NO regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 269, 26066–26075 (1994).
Violi, F., Marino, R., Milite, M. T. & Loffredo, L. NO and its role in lipid peroxidation. Diabetes Metab. Res. Rev. 15, 283–288 (1999).
Moore, K. P., Darley-Usmar, V., Morrow, J. & Roberts, L. J. Formation of F2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circ. Res. 77, 335–341 (1995).
Batthyany, C. et al. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 281, 20450–20463 (2006).
Wright, M. M. et al. Fatty acid transduction of NO signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc. Natl Acad. Sci. USA 103, 4299–4304 (2006).
Milstien, S. & Katusic, Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 263, 681–684 (1999).
Kuzkaya, N., Weissmann, N., Harrison, D. G. & Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 278, 22546–22554 (2003).
Forstermann, U. & Munzel, T. Endothelial NO synthase in vascular disease: from marvel to menace. Circulation 113, 1708–1714 (2006).
Kirsch, M. & de Groot, H. Reaction of peroxynitrite with reduced nicotinamide nucleotides, the formation of hydrogen peroxide. J. Biol. Chem. 274, 24664–24670 (1999).
Goldstein, S. & Czapski, G. Reactivity of peroxynitrite versus simultaneous generation of (*)NO and O(2)(*)(−) toward NADH. Chem. Res. Toxicol. 13, 736–741 (2000).
Ischiropoulos, H. et al. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 298, 431–437 (1992). The first article on peroxynitrite-induced tyrosine nitration.
MacMillan-Crow, L. A., Crow, J. P. & Thompson, J. A. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 37, 1613–1622 (1998).
Alvarez, B. et al. Inactivation of human Cu, Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic. Biol. Med. 37, 813–822 (2004).
Beckman, J. S., Estevez, A. G., Crow, J. P. & Barbeito, L. Superoxide dismutase and the death of motoneurons in ALS. Trends Neurosci. 24 (Suppl. 11), 15–20 (2001).
Savvides, S. N. et al. Crystal structure of the antioxidant enzyme glutathione reductase inactivated by peroxynitrite. J. Biol. Chem. 277, 2779–2784 (2002).
Aykac-Toker, G., Bulgurcuoglu, S. & Kocak-Toker, N. Effect of peroxynitrite on glutaredoxin. Hum. Exp. Toxicol. 20, 373–376 (2001).
Hogg, N., Darley-Usmar, V. M., Wilson, M. T. & Moncada, S. The oxidation of α-tocopherol in human low-density lipoprotein by the simultaneous generation of superoxide and NO. FEBS Lett. 326, 199–203 (1993).
Van der Vliet, A. et al. Interactions of peroxynitrite with human plasma and its constituents: oxidative damage and antioxidant depletion. Biochem. J. 303, 295–301 (1994).
Vatassery, G. T., Lai, J. C., DeMaster, E. G., Smith, W. E. & Quach, H. T. Oxidation of vitamin E and vitamin C and inhibition of brain mitochondrial oxidative phosphorylation by peroxynitrite. J. Neurosci. Res. 75, 845–853 (2004).
Hausladen, A. & Fridovich, I. Superoxide and peroxynitrite inactivate aconitases, but NO does not. J. Biol. Chem. 269, 29405–29408 (1994).
Crow, J. P., Beckman, J. S. & McCord, J. M. Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochemistry 34, 3544–3552 (1995).
Mohr, S, Stamler, J. S. & Brune, B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by NO, peroxynitrite and related nitrosating agents. FEBS Lett. 348, 223–227 (1994).
Konorev, E. A., Hogg, N. & Kalyanaraman, B. Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett. 427, 171–174 (1998).
Mihm, M. J. et al. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc. Res. 49, 798–807 (2001).
Blanchard-Fillion, B. et al. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J. Biol. Chem. 276, 46017–46023 (2001).
Paxinou, E. et al. Induction of α-synuclein aggregation by intracellular nitrative insult. J. Neurosci. 21, 8053–8061 (2001).
Reynolds, M. R., Lukas, T. J., Berry, R. W. & Binder, L. I. Peroxynitrite-mediated tau modifications stabilize preformed filaments and destabilize microtubules through distinct mechanisms. Biochemistry 45, 4314–4326 (2006).
Okamoto, T. et al. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 276, 29596–29602 (2001).
Migita, K. et al. Peroxynitrite-mediated matrix metalloproteinase-2 activation in human hepatic stellate cells. FEBS Lett. 579, 3119–3125 (2005).
Ji, Y., Neverova, I., Van Eyk, J. E. & Bennett, B. M. Nitration of tyrosine 92 mediates the activation of rat microsomal glutathione S-transferase by peroxynitrite. J. Biol. Chem. 281, 986–991 (2006).
Xie, Z. et al. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J. Biol. Chem. 281, 6366–6375 (2005).
Jang, B. & Han, S. Biochemical properties of cytochrome c nitrated by peroxynitrite. Biochimie 88, 53–58 (2006).
Bauer, M. L., Beckman, J. S., Bridges, R. J., Fuller, C. M. & Matalon, S. Peroxynitrite inhibits sodium uptake in rat colonic membrane vesicles. Biochim. Biophys. Acta 1104, 87–94 (1992).
Hu, P., Ischiropoulos, H., Beckman, J. S. & Matalon, S. Peroxynitrite inhibition of oxygen consumption and sodium transport in alveolar type II cells. Am. J. Physiol. 266, L628–L634 (1994).
Klebl, B. M., Ayoub, A. T. & Pette, D. Protein oxidation, tyrosine nitration, and inactivation of sarcoplasmic reticulum Ca2+-ATPase in low-frequency stimulated rabbit muscle. FEBS Lett. 422, 381–384 (1998).
Viner, R. I., Williams, T. D., Schoneich, C. NO-dependent modification of the sarcoplasmic reticulum Ca-ATPase: localization of cysteine target sites. Free Radic. Biol. Med. 29, 489–496 (2000).
Grover, A. K., Kwan, C. Y. & Samson, S. E. Effects of peroxynitrite on sarco/endoplasmic reticulum Ca2+ pump isoforms SERCA2b and SERCA3a. Am. J. Physiol. Cell Physiol. 285, C1537–C1543 (2003).
Chakraborti, S., Mandal, A., Das, S. & Chakraborti, T. Inhibition of Na+/Ca2+ exchanger by peroxynitrite in microsomes of pulmonary smooth muscle: role of matrix metalloproteinase-2. Biochim. Biophys. Acta 1671, 70–78 (2004).
Gutierrez-Martin, Y. et al. Alteration of cytosolic free calcium homeostasis by SIN-1: high sensitivity of L-type Ca2+ channels to extracellular oxidative/nitrosative stress in cerebellar granule cells. J. Neurochem. 92, 973–989 (2005).
Zou, M., Yesilkaya, A. & Ullrich, V. Peroxynitrite inactivates prostacyclin synthase by heme-thiolate-catalyzed tyrosine nitration. Drug Metab. Rev. 31, 343–349 (1999).
Bagnasco, P. et al. Peroxynitrite modulates acidic fibroblast growth factor (FGF-1) activity. Arch. Biochem. Biophys. 419, 178–189 (2003).
Freels, J. L. et al. Enhanced activity of human IL-10 after nitration in reducing human IL-1 production by stimulated peripheral blood mononuclear cells. J. Immunol. 169, 4568–4571 (2002).
van der Vliet, A, Hristova, M., Cross, C. E., Eiserich, J. P. & Goldkorn, T. Peroxynitrite induces covalent dimerization of epidermal growth factor receptors in A431 epidermoid carcinoma cells. J. Biol. Chem. 273, 31860–31866 (1998).
Lewis, S. J., Hoque, A., Walton, T. M. & Kooy, N. W. Potential role of nitration and oxidation reactions in the effects of peroxynitrite on the function of β-adrenoceptor sub-types in the rat. Eur. J. Pharmacol. 518, 187–194 (2005).
Shibuya, A. et al. Nitration of PPARγ inhibits ligand-dependent translocation into the nucleus in a macrophage-like cell line, RAW 264. FEBS Lett. 525, 43–47 (2002).
Giuntini, J., Giusti, L., Lucacchini, A. & Mazzoni, M. R. Modulation of A1 adenosine receptor signaling by peroxynitrite. Biochem. Pharmacol. 67, 375–383 (2004).
Nomiyama, T. et al. Reduction of insulin-stimulated glucose uptake by peroxynitrite is concurrent with tyrosine nitration of insulin receptor substrate-1. Biochem. Biophys. Res. Commun. 320, 639–647 (2004).
Clavreul, N. et al. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 20, 518–520 (2006).
Newman, D. K. et al. Nitration of PECAM-1 ITIM tyrosines abrogates phosphorylation and SHP-2 binding. Biochem. Biophys. Res. Commun. 296, 1171–1179 (2002).
Bar-Shai, M. & Reznick, A. Z. Peroxynitrite induces an alternative NF-κB activation pathway in l8 rat myoblasts. Antioxid. Redox Signal. 8, 639–652 (2006).
Zouki, C., Jozsef, L., Ouellet, S., Paquette, Y. & Filep, J. G. Peroxynitrite mediates cytokine-induced IL-8 gene expression and production by human leukocytes. J. Leukoc. Biol. 69, 815–824 (2001).
Matata, B. M. & Galinanes, M. Peroxynitrite is an essential component of cytokine production mechanism in human monocytes through modulation of NFκB DNA-binding activity. J. Biol. Chem. 277, 2330–2335 (2001).
Levrand, S. et al. Peroxynitrite is a potent inhibitor of NFκB activation triggered by inflammatory stimuli in cardiac and endothelial cell lines. J. Biol. Chem. 280, 4878–4887 (2005).
Knapp, L. T., Kanterewicz, B. I., Hayes, E. L. & Klann, E. Peroxynitrite-induced tyrosine nitration and inhibition of protein kinase C. Biochem. Biophys. Res. Commun. 286, 764–770 (2001).
Bapat, S., Verkleij, A. & Post, J. A. Peroxynitrite activates mitogen-activated protein kinase (MAPK) via a MEK-independent pathway: a role for protein kinase C. FEBS Lett. 499, 21–26 (2001).
Mallozzi, C., Di Stasi, A. M. & Minetti, M. Nitrotyrosine mimics phosphotyrosine binding to the SH2 domain of the src family tyrosine kinase lyn. FEBS Lett. 503, 189–195 (2001).
Mallozzi, C., Di Stasi, M. A & Minetti, M. Peroxynitrite-dependent activation of src tyrosine kinases lyn and hck in erythrocytes is under mechanistically different pathways of redox control. Free Radic. Biol. Med. 30, 1108–1117 (2001).
Yuen, E. C., Gunther, E. C. & Bothwell, M. NO activation of TrkB through peroxynitrite. Neuroreport 11, 3593–3597 (2000).
Platt, D. H. et al. Peroxynitrite increases VEGF expression in vascular endothelial cells via STAT3. Free Radic. Biol. Med. 39, 1353–1361 (2005).
Klotz, L. O., Schieke, S. M., Sies, H. & Holbrook, N. J. Peroxynitrite activates the phosphoinositide 3-kinase/Akt pathway in human skin primary fibroblasts. Biochem. J. 352, 219–225 (2000).
Shacka, J. J. et al. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 13, 1506–1514 (2006).
Zingarelli, B., Salzman, A. L. & Szabó, C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ. Res. 83, 85–94 (1998).
Haddad, I. Y. et al. Concurrent generation of NO and superoxide damages surfactant protein Am. J. Physiol. 267, L242–L249 (1994).
Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and NO. Arch. Biochem. Biophys. 288, 481–487 (1991). This is the first paper supporting the reactions of peroxynitrite-derived radicals in hydrophobic compartments.
Botti, H., Trostchansky, A., Batthyany, C. & Rubbo, H. Reactivity of peroxynitrite and NO with LDL. IUBMB Life 57, 407–412 (2005).
Szabó, C., Zingarelli, B., O'Connor, M. & Salzman, A. L. DNA strand breakage, activation of poly-ADP ribosyl synthetase, and cellular energy depletion are involved in the cytotoxicity in macrophages and smooth muscle cells exposed to peroxynitrite. Proc. Natl Acad. Sci. USA 93, 1753–1758 (1996). The first report linking peroxynitrite to the activation of the nuclear enzyme PARP and subsequent cell death.
Brown, G. C. & Borutaite, V. Inhibition of mitochondrial respiratory complex I by NO, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta 1658, 44–49 (2004).
Shiva, S. et al. Nitroxia: the pathological consequence of dysfunction in the NO-cytochrome c oxidase signaling pathway. Free Radic. Biol. Med. 38, 297–306 (2005).
Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 140–141, 105–112 (2003).
Radi, R., Cassina, A., Hodara, R., Quijano, C. & Castro, L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 33, 1451–1464 (2002).
Quijano, C., Cassina, A., Castro, L., Rodriguez, M. & Radi, R. in Nitric Oxide, Cell Signaling and Gene Expression (eds Lamas, S. & Cadenas, E.) (CRC, Boca Raton, 2005).
Ghafourifar, P. & Cadenas, E. Mitochondrial NO synthase. Trends Pharmacol. Sci. 26, 190–195 (2005).
Lacza, Z. et al. Mitochondrial NO and reactive nitrogen species production: does mtNOS exist? Nitric Oxide 14, 162–168 (2006).
Lacza, Z. et al. Mitochondria produce reactive nitrogen species via an arginine-independent pathway. Free Radic. Res. 40, 369–378 (2006).
Radi, R., Rodriguez, M., Castro, L. & Telleri, R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 308, 89–95 (1994). An early report demonstrating the ability of peroxynitrite to promote mitochondrial dysfunction.
Riobo, N. A. et al. NO inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem. J. 359, 139–145 (2001).
Boczkowski, J. et al. Peroxynitrite-mediated mitochondrial dysfunction. Biol. Signals Recept. 10, 66–80 (2001).
Cassina, A. M. et al. Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 275, 21409–21415 (2000).
Batthyany, C. et al. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry 44, 8038–8046 (2005).
Giulivi, C., Poderoso, J. J. & Boveris, A. Production of nitric oxide by mitochondria. J. Biol. Chem. 273, 11038–11043 (1998).
MacMillan-Crow, L. A. et al. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl Acad. Sci. USA 93, 11853–11858 (1996).
Yamakura, F., Taka, H., Fujimura, T. & Murayama, K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 273, 14085–14089 (1998).
Quijano, C. et al. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem. 276, 11631–11638 (2001).
Virag, L. & Szabó, C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 54, 375–429 (2002).
Jagtap, P. & Szabó, C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nature Rev. Drug Discov. 4, 421–440 (2005).
Bolanos, J. P., Heales, S. J., Land, J. M. & Clark, J. B. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J. Neurochem. 64, 1965–1972 (1995).
Szabó, C. & Salzman, A. L. Endogenous peroxynitrite is involved in the inhibition of cellular respiration in immuno-stimulated J774.2 macrophages. Biochem. Biophys. Res. Comm. 209, 739–743 (1995). An early report implying the role of endogenously produced peroxynitrite in inflammatory- and immune-mediated cell injury.
Szabó, C., Zingarelli, B. & Salzman, A. L. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous NO and peroxynitrite. Circ. Res. 78, 1051–1063 (1996).
Suzuki, Y. et al. Na+, K+-ATPase activity is inhibited in cultured intestinal epithelial cells by endotoxin or NO. Int. J. Mol. Med. 15, 871–877 (2005).
Sacksteder C. A. et al. Endogenously nitrated proteins in mouse brain: links to neurodegenerative disease. Biochemistry 45, 8009–8022 (2006).
Nin, N. et al. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible NO synthase inhibition. Int. Care Med. 30, 2271–2278 (2004).
Pacher, P., Beckman, J. S. & Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 (2007).
Balafanova, Z. et al. Nitric oxide (NO) induces nitration of protein kinase C epsilon (PKCε), facilitating PKCε translocation via enhanced PKCε-RACK2 interactions: a novel mechanism of no-triggered activation of PKCε. J Biol. Chem. 277, 15021–15027 (2002).
Pehar, M. et al. Peroxynitrite transforms nerve growth factor into an apoptotic factor for motor neurons. Free Radic. Biol. Med. 41, 1632–1644 (2006).
Lanone, S. et al. Inducible NO synthase (NOS2) expressed in septic patients is nitrated on selected tyrosine residues: implications for enzymic activity. Biochem. J. 366, 399–404 (2002).
Zouki, C., Zhang, S. L., Chan, J. S. & Filep, J. G. Peroxynitrite induces integrin-dependent adhesion of human neutrophils to endothelial cells via activation of the Raf-1/MEK/Erk pathway. FASEB J. 15, 25–27 (2001).
Landino, L. M., Crews, B. C., Timmons, M. D., Morrow, J. D. & Marnett, L. J. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc. Natl Acad. Sci. USA 93, 15069–15074 (1996).
Trostchansky, A. et al. Interactions between nitric oxide and peroxynitrite during prostaglandin endoperoxide H synthase-1 catalysis: a free radical mechanism of inactivation. Free Radic. Biol. Med. 42, 1029–1038 (2007).
Ito, K., Hanazawa, T., Tomita, K., Barnes, P. J. & Adcock, I. M. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem. Biophys. Res. Commun. 315, 240–245 (2004).
Levonen, A. L. et al. Mechanisms of cell signaling by NO and peroxynitrite: from mitochondria to MAP kinases. Antioxid. Redox Signal. 3, 215–229 (2001).
Sohn, H. Y. et al. Crucial role of local peroxynitrite formation in neutrophil-induced endothelial cell activation. Cardiovasc. Res. 57, 804–815 (2003).
Kerry, N. & Rice-Evans, C. Peroxynitrite oxidises catechols to o-quinones. FEBS Lett. 437, 167–171 (1998).
Macarthur, H., Westfall, T. C., Riley, D. P., Misko, T. P. & Salvemini, D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc. Natl Acad. Sci. USA 97, 9753–9758 (2000).
Heijnen, H. F. G. et al. Subcellular localization of tyrosine-nitrated proteins is dictated by reactive oxygen species generating enzymes and by proximity to nitric oxide synthase. Free Radic. Biol. Med. 40, 1903–1913 (2006).
Fries, D. M. et al. Expression of inducible nitric oxide synthase and intracellular protein tyrosine nitration in vascular smooth muscle cells: role of reactive oxygen species. J. Biol. Chem. 278, 22901–22907 (2003).
Giasson, B. I. et al., Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 (2000).
Birnboim, H. C., Lemay, A. M., Lam, D. K., Goldstein, R. & Webb, J. R. MHC class II-restricted peptides containing the inflammation-associated marker 3-nitrotyrosine evade central tolerance and elicit a robust cell-mediated immune response. J. Immunol. 171, 528–532 (2003).
Herzog, J., Maekawa, Y., Cirrito, T. P., Illian, B. S. & Unanue, E. R. Activated antigen-presenting cells select and present chemically modified peptides recognized by unique CD4 T cells. Proc. Natl Acad. Sci. USA 102, 7928–7933 (2005).
Salvemini, D., Doyle, T. M. & Cuzzocrea, S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem. Soc. Trans. 34, 965–970 (2006).
Altug, S. et al. Biological time-dependent difference in effect of peroxynitrite demonstrated by the mouse hot plate pain model. Chronobiol. Int. 23, 583–591 (2006).
Khattab, M. M. Tempol, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur. J. Pharmacol. 548, 167–173 (2006).
Bezerra, M. M. et al. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch. Pharmacol. 374, 265–273 (2007).
Wang, Z. Q. et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 309, 869–878 (2004).
Hooper, D. C. et al. The central nervous system inflammatory response to neurotropic virus infection is peroxynitrite dependent. J. Immunol. 167, 3470–3477 (2001).
Padalko, E. Peroxynitrite inhibition of Coxsackievirus infection by prevention of viral RNA entry. Proc. Natl Acad. Sci. USA 101, 11731–11736 (2004).
Virag, L., Szabó, E., Gergely, P. & Szabó, C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol. Lett. 140–141, 113–124 (2003).
Virag, L., Marmer, D. J. & Szabó, C. Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation. Free Radic. Biol. Med. 25, 1075–1082 (1998).
Zhuang, S. & Simon, G. Peroxynitrite-induced apoptosis involves activation of multiple caspases in HL-60 cells. Am. J. Physiol. Cell Physiol. 279, C341–C351 (2000).
Vicente, S. et al. NO and peroxynitrite induce cellular death in bovine chromaffin cells: evidence for a mixed necrotic and apoptotic mechanism with caspases activation. J. Neurosci. Res. 84, 78–96 (2006).
Borutaite, V., Morkuniene, R. & Brown, G. C. Release of cytochrome c from heart mitochondria is induced by high Ca2+ and peroxynitrite and is responsible for Ca(2+)-induced inhibition of substrate oxidation. Biochim. Biophys. Acta 1453, 41–48 (1999).
Richter, C., Schweizer, M. & Ghafourifar P. Mitochondria, NO, and peroxynitrite. Methods Enzymol. 301, 381–393 (1999).
Estevez, E. et al. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J. Neurosci. 18, 923–931 (1998).
Virag, L. et al. Requirement of intracellular calcium mobilization for peroxynitrite-induced poly(ADP-ribose) synthetase activation and cytotoxicity. Mol. Pharmacol. 56, 824–833 (1999).
Dickhout, J. G. et al. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 25, 2623–2629 (2005).
Redondo, P. C. et al. Hydrogen peroxide and peroxynitrite enhance Ca2+ mobilization and aggregation in platelets from type 2 diabetic patients. Biochem. Biophys. Res. Commun. 333, 794–802 (2005).
Whiteman, M. et al. Peroxynitrite mediates calcium-dependent mitochondrial dysfunction and cell death via activation of calpains. FASEB J. 18, 1395–1397 (2004).
Cantoni, O. et al. Survival pathways triggered by peroxynitrite in cells belonging to the monocyte/macrophage lineage. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 14, 118–123 (2005).
Zhang, X. et al. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem. 82, 181–191 (2002).
Dawson, V. L. & Dawson, T. M. Deadly conversations: nuclear-mitochondrial cross-talk. J. Bioenerg. Biomembr. 36, 287–294 (2004).
Beller, C. J. et al. Activation of the peroxynitrite-poly(adenosine diphosphate-ribose) polymerase pathway during neointima proliferation: a new target to prevent restenosis after endarterectomy. J. Vasc. Surg. 43, 824–830 (2006).
Tao, L. et al. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation 114, 1395–1402 (2006).
Sappington, P. L. et al. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 123, 790–802 (2002).
Ulloa, L. & Messmer, D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 17, 189–201 (2006).
Dixit, K. & Moinuddin, A. A. Immunological studies on peroxynitrite modified human DNA. Life Sci. 77, 2626–2642 (2005).
Ohmori, H. & Kanayama, N. Immunogenicity of an inflammation-associated product, tyrosine nitrated self-proteins. Autoimmun. Rev. 4, 224–229 (2005).
Radi, R., Denicola, A., Alvarez, B., Ferrer-Sueta, G. & Rubbo, H. in Nitric Oxide: Biology and Pathobiology Ch.4 (ed. Ignarro, L. J.) 57–82 (Academic Press, San Diego, 2000).
Bryk, R., Griffin, P. & Nathan, C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 (2000).
Trujillo, M. et al. Trypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J. Biol. Chem. 279, 34175–34182 (2004).
Dubuisson, M. et al. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett. 571, 161–165 (2004).
Rhee, S. G., Chae, H. Z. & Kim, K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 38, 1543–1552 (2005).
Kooy, N. W., Royall, J. A., Ischiropoulos, H. & Beckman, J. S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 16, 149–156 (1994). The first article describing the ability of urate to attenuate peroxynitrite-induced oxidative reactions in vitro.
Whiteman, M., Ketsawatsakul, U. & Halliwell, B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann. N. Y. Acad. Sci. 962, 242–259 (2002).
Scott, G. S. et al. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc. Natl Acad. Sci. USA. 99 16303–16308 (2002).
Robinson, K. M., Morre, J. T. & Beckman, J. S. Triuret: a novel product of peroxynitrite-mediated oxidation of urate. Arch. Biochem. Biophys. 423, 213–217 (2004).
Scott, G. S. et al. Uric acid protects against secondary damage after spinal cord injury. Proc. Natl Acad. Sci. USA 102, 3483–3488 (2005).
Szabó, C. et al. Mercaptoethylguanidine and guanidine inhibitors of nitric-oxide synthase react with peroxynitrite and protect against peroxynitrite-induced oxidative damage. J. Biol. Chem. 272, 9030–9036 (1997). The characterization of mercaptoalkylguanidines as compounds of mixed pharmacological action: iNOS inhibition and peroxynitrite scavenging.
Zingarelli, B., Ischiropoulos, H., Salzman, A. L. & Szabó, C. Amelioration by mercaptoethylguanidine of the vascular and energetic failure in haemorrhagic shock in the anesthetised rat. Eur. J. Pharmacol. 338, 55–65 (1997).
Moochhala, S. M. et al. Mercaptoethylguanidine inhibition of inducible NO synthase and cyclooxygenase-2 expressions induced in rats after fluid-percussion brain injury. J. Trauma 59, 450–457 (2005).
Ploner, F. et al. Effects of combined selective iNOS inhibition and peroxynitrite blockade during endotoxemia in pigs. Shock 16, 130–136 (2001).
Lancel, S. et al. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J. Am. Coll. Cardiol. 43, 2348–2358 (2004).
Klotz, L. O. & Sies, H. Defenses against peroxynitrite: selenocompounds and flavonoids. Toxicol. Lett. 140–141, 125–132 (2003).
Sugiura, H. et al. Role of peroxynitrite in airway microvascular hyperpermeability during late allergic phase in guinea pigs. Am. J. Respir. Crit. Care Med. 160, 663–671 (1999).
Daiber, A., Zou, M. H., Bachschmid, M. & Ullrich, V. Ebselen as a peroxynitrite scavenger in vitro and ex vivo. Biochem. Pharmacol. 59, 153–160 (2000).
Noiri, E. et al. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal Physiol. 281, F948–F957 (2001).
Gealekman, O. et al. Endothelial dysfunction as a modifier of angiogenic response in Zucker diabetic fat rat: amelioration with ebselen. Kidney Int. 66, 2337–2347 (2004).
Arteel, G. E., Briviba, K. & Sies, H. Protection against peroxynitrite. FEBS Lett. 445, 226–230 (1999).
Parnham, M. & Sies, H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opin. Investig. Drugs. 9, 607–619 (2000).
Sies, H., Sharov, V. S., Klotz, L. O. & Briviba, K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. J. Biol. Chem. 272, 27812–24817 (1997).
Arteel, G. E., Mostert, V., Oubrahim, H., Briviba, K., Abel, J. & Sies, H. Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol. Chem. 379, 1201–1205 (1998).
Briviba, K., Roussyn, I., Sharov, V. S. & Sies, H. Attenuation of oxidation and nitration reactions of peroxynitrite by selenomethionine, selenocystine and ebselen. Biochem. J. 319, 13–15 (1996).
Jacob, C., Arteel, G. E., Kanda, T., Engman, L. & Sies, H. Sulfur, selenium, tellurium: protection by organotellurium compounds against peroxynitrite-mediated oxidation and nitration reactions. Biochem. Pharmacol. 55, 817–823 (1998).
Fernandes, D. C., Medinas, D. B., Alves, M. J. & Augusto, O. Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic. Biol. Med. 38, 189–200 (2005).
Bonini, M. G., Mason, R. P. & Augusto, O. The mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species. Chem. Res. Toxicol. 15, 506–511 (2002).
Thiemermann, C., McDonald, M. C. & Cuzzocrea, S. The stable nitroxide, tempol, attenuates the effects of peroxynitrite and oxygen-derived free radicals. Crit. Care Med. 29, 223–224 (2001).
Cuzzocrea, S. et al. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 875, 96–106 (2000).
Sepodes, B. et al. Tempol, an intracelullar free radical scavenger, reduces liver injury in hepatic ischemia-reperfusion in the rat. Transplant Proc. 36, 849–853 (2004).
Isobe, C. et al. Cabergoline scavenges peroxynitrite enhanced by L-DOPA therapy in patients with Parkinson's disease. Eur. J. Neurol. 13, 346–350 (2006).
Rork, T. H., Hadzimichalis, N. M., Kappil, M. A. & Merrill, G. F. Acetaminophen attenuates peroxynitrite-activated matrix metalloproteinase-2-mediated troponin I cleavage in the isolated guinea pig myocardium. J. Mol. Cell. Cardiol. 40, 553–561 (2006).
Mason, R. P., Kalinowski, L., Jacob, R. F., Jacoby, A. M. & Malinski, T. Nebivolol reduces nitroxidative stress and restores NO bioavailability in endothelium of black Americans. Circulation 112, 3795–3801 (2005).
Daiber, A. et al. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem. Biophys. Res. Commun. 338, 1865–1874 (2005).
Fernandes, E., Gomes, A., Costa, D. & Lima, J. L. Pindolol is a potent scavenger of reactive nitrogen species. Life Sci. 77, 1983–1992 (2005).
Coffey, M. J., Phare, S. M. & Peters-Golden, M. Peroxynitrite-induced nitrotyrosination of proteins is blocked by direct 5-lipoxygenase inhibitor zileuton. J. Pharmacol. Exp. Ther. 299, 198–203 (2001).
Cabassi, A. et al. Effects of chronic N-acetylcysteine treatment on the actions of peroxynitrite on aortic vascular reactivity in hypertensive rats. J. Hypertens. 19, 1233–1244 (2001).
Althaus, J. S. et al. Structure activity relationships of peroxynitrite scavengers an approach to NO neurotoxicity. Res. Commun. Chem. Pathol. Pharmacol. 83, 243–254 (1994).
Selley, M. L. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 1037, 1–6 (2005).
Banno, M. et al. The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology 48, 283–290 (2005).
Acquaviva, R. et al. Propofol attenuates peroxynitrite-mediated DNA damage and apoptosis in cultured astrocytes: an alternative protective mechanism. Anesthesiology 101, 1363–1371 (2004).
Trujillo, M. et al. Peroxynitrite-derived carbonate and nitrogen dioxide radicals readily react with lipoic and dihydrolipoic acid. Free Radic. Biol. Med. 39, 279–288 (2005).
Lee, C. S. et al. Effect of R-(-)-deprenyl and harmaline on dopamine- and peroxynitrite-induced membrane permeability transition in brain mitochondria. Neurochem. Res. 27, 215–224 (2002).
Maruyama, W., Takahashi, T., Youdim, M. & Naoi, M. The anti-Parkinson drug, rasagiline, prevents apoptotic DNA damage induced by peroxynitrite in human dopaminergic neuroblastoma SH-SY5Y cells. J. Neural Transm. 109, 467–481 (2002).
Lopez-Alarcon, C. et al. Reactivity of 1,4-dihydropyridines toward SIN-1-derived peroxynitrite. Pharm. Res. 21, 1750–1757 (2004).
Bartesaghi, S. et al. Reactions of desferrioxamine with peroxynitrite-derived carbonate and nitrogen dioxide radicals. Free Radic. Biol. Med. 36, 471–483 (2004).
Oury, T. D. et al., Cold-induced brain edema in mice. Involvement of extracellular superoxide dismutase and NO. J. Biol. Chem. 268, 15394–15398 (1993).
Ye, Y. et al. Prevention of peroxynitrite-induced apoptosis of motor neurons and pc12 cells by tyrosine-containing peptides. J. Biol. Chem. 282, 6324–6337 (2007).
Panasenko, O. M., Sharov, V. S., Briviba, K. & Sies, H. Interaction of peroxynitrite with carotenoids in human low density lipoproteins. Arch. Biochem. Biophys. 373, 302–305 (2000).
Arteel, G. E. & Sies, H. Protection against peroxynitrite by cocoa polyphenol oligomers. FEBS Lett. 462, 167–170 (1999).
Schroeder, P., Klotz, L. O. & Sies, H. Amphiphilic properties of (-)-epicatechin and their significance for protection of cells against peroxynitrite. Biochem. Biophys. Res. Commun. 307, 69–73 (2003).
Ferrer-Sueta, G., Quijano, C., Alvarez, B. & Radi, R. Reactions of manganese porphyrins and manganese-superoxide dismutase with peroxynitrite. Methods Enzymol. 349, 23–37 (2002).
Stern, M. K., Jensen, M. P. & Kramer, K. Peroxynitrite decomposition catalysts. J. Am. Chem. Soc. 118, 8735–8736 (1996). The first paper on the synthesis and initial characterization of a peroxynitrite decomposition catalyst ferro-porphyrinic compound.
Jensen, M. P. & Riley, D. P. Peroxynitrite decomposition activity of iron porphyrin complexes. Inorg. Chem. 41, 4788–4797 (2002).
Shimanovich, R. & Groves, J. T. Mechanisms of peroxynitrite decomposition catalyzed by FeTMPS, a bioactive sulfonated iron porphyrin. Arch. Biochem. Biophys. 387, 307–317 (2001).
Salvemini, D. et al. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 303, 217–220 (1996).
Salvemini, D., Wang, Z. Q., Stern, M. K., Currie, M. G. & Misko, T. P. Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc. Natl Acad. Sci. USA 95, 2659–2663 (1998). An initial characterization of the pharmacological effect of molecules designed to catalytically neutralize peroxynitrite.
Cross, A. H. et al. A catalyst of peroxynitrite decomposition inhibits murine experimental autoimmune encephalomyelitis. J. Neuroimmunol. 107, 21–28 (2001).
Muscoli, C. et al. Peroxynitrite decomposition catalyst prevents apoptotic cell death in a human astrocytoma cell line incubated with supernatants of HIV-infected macrophages. BMC Neurosci. 3, 13 (2002).
Cuzzocrea, S. et al. Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J. 14, 1061–1072 (2000).
Thiyagarajan, M., Kaul, C. L. & Sharma, S. S. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br. J. Pharmacol. 142, 899–911 (2004).
Sharma, S. S., Munusamy, S., Thiyagarajan, M. & Kaul, C. L. Neuroprotective effect of peroxynitrite decomposition catalyst and poly(adenosine diphosphate-ribose) polymerase inhibitor alone and in combination in rats with focal cerebral ischemia. J. Neurosurg. 101, 669–675 (2004).
Xie, Z. et al. Peroxynitrite mediates neurotoxicity of amyloid β-peptide1–42- and lipopolysaccharide-activated microglia. J. Neurosci. 22, 3484–3492 (2002).
Mander, P. & Brown, G. C. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J. Neuroinflammation 22, 20 (2005).
Tan, K. H., Harrington, S., Purcell, W. M. & Hurst, R. D. Peroxynitrite mediates NO-induced blood–brain barrier damage. Neurochem. Res. 29, 579–587 (2004).
Ferdinandy, P., Danial, H. Ambrus, I., Rothery, R. A. & Schulz, R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ. Res. 87, 241–247 (2000).
Kim, J. Y., Lee, K. H., Lee, B. K. & Ro, J. Y. Peroxynitrite modulates release of inflammatory mediators from guinea pig lung mast cells activated by antigen–antibody reaction. Int. Arch. Allergy Immunol. 137, 104–114 (2005).
Beauchamp, M. H. et al. Redox-dependent effects of NO on microvascular integrity in oxygen-induced retinopathy. Free Radic. Biol. Med. 37, 1885–1894 (2004).
Chirino, Y. I., Hernandez-Pando, R. & Pedraza-Chaverri, J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 4, 20 (2004).
Nangle, M. R., Cotter, M. A. & Cameron, N. E. Effects of the peroxynitrite decomposition catalyst, FeTMPyP, on function of corpus cavernosum from diabetic mice. Eur. J. Pharmacol. 502, 143–148 (2004).
Cuzzocrea, S. et al. A role for NO-mediated peroxynitrite formation in a model of endotoxin induced shock. J. Pharmacol. Exp. Ther. 319, 73–81 (2006).
Szabó, C. et al. Pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol. Med. 8, 571–580 (2002). A description of an iron-porphyrinic peroxynitrite decomposition calalyst: anti-inflammatory effects in vivo.
Tauskela, J. S. et al. Competing approaches to excitotoxic neuroprotection by inert and catalytic antioxidant porphyrins. Neurosci. Lett. 401, 236–241 (2006).
Obrosova, I. G. et al. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 19, 401–403 (2005).
Sugawara, R. et al. Peroxynitrite decomposition catalyst, FP15, and poly(ADP-ribose) polymerase inhibitor, PJ34, inhibit leukocyte entrapment in the retinal microcirculation of diabetic rats. Curr. Eye Res. 29, 11–16 (2004).
Bianchi, C. et al. A novel peroxynitrite decomposer catalyst (FP-15) reduces myocardial infarct size in an in vivo peroxynitrite decomposer and acute ischemia-reperfusion in pigs. Ann. Thorac. Surg. 74, 1201–1207 (2002).
Pacher, P. et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107, 896–904 (2003).
Naidu, B. V. et al. Enhanced peroxynitrite decomposition protects against experimental obliterative bronchiolitis. Exp. Mol. Pathol. 75, 12–17 (2003).
Lacza, Z. et al. PARP inhibition improves the effectiveness of neural stem cell transplantation in experimental brain trauma. Int. J. Mol. Med. 12, 153–159 (2003).
Naidu, B. V. et al. Critical role of reactive nitrogen species in lung ischemia-reperfusion injury. J. Heart Lung Transplant. 22, 784–793 (2003).
Mabley, J. G. et al. Suppression of intestinal polyposis in Apcmin/+ mice by targeting the NO or poly(ADP-ribose) pathways. Mutat. Res. 548, 107–116 (2004).
Mabley, J. G. et al. Beneficial effects of the peroxynitrite decomposition catalyst FP15 in murine models of arthritis and colitis. Mol. Med. 8, 581–590 (2002).
Pieper, G. M. et al. Protective mechanisms of a metalloporphyrinic peroxynitrite decomposition catalyst, WW85, in rat cardiac transplants. J. Pharmacol. Exp. Ther. 314, 53–60 (2005).
Mangino, M. J. et al. Role of peroxynitrite anion in renal hypothermic preservation injury. Transplantation 80, 1455–1460 (2005).
Szabó, C., Day, B. J. & Salzman, A. L. Evaluation of the relative contribution of NO and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 381, 82–86 (1996).
Zingarelli, B., Day, B. J., Crapo, J. D., Salzman, A. L. & Szabó, C. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br. J. Pharmacol. 120, 259–267 (1997).
Cuzzocrea, S., Zingarelli, B., Costantino, G. & Caputi, A. P. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-induced pleurisy. Free Radic. Biol. Med. 26, 25–33 (1999).
Kastenbauer, S., Koedel, U., Becker, B. F. & Pfister, H. W. Pneumococcal meningitis in the rat: evaluation of peroxynitrite scavengers for adjunctive therapy. Eur. J. Pharmacol. 449, 177–181 (2002).
Liu, D., Bao, F., Prough, D. S. & Dewitt, D. S. Peroxynitrite generated at the level produced by spinal cord injury induces peroxidation of membrane phospholipids in normal rat cord: reduction by a metalloporphyrin. J. Neurotrauma. 22, 1123–1133 (2005).
Ferrer-Sueta, G. et al. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J. Biol. Chem. 278, 27432–27438 (2003). A detailed biochemical characterization of the peroxynitrite/manganese porphyrin interactions.
Trostchansky, A. et al. Peroxynitrite flux-mediated LDL oxidation is inhibited by manganese porphyrins in the presence of uric acid. Free Radic. Biol. Med. 35, 1293–1300 (2003).
Crow, J. P. Peroxynitrite scavenging by metalloporphyrins and thiolates. Free Radic. Biol. Med. 28, 1487–1494 (2000).
Ferrer-Sueta, G., Hannibal, L., Batinic-Haberle, I. & Radi, R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic. Biol. Med. 41, 503–512 (2006).
Faulkner, K. M., Liochev, S. I. & Fridovich, I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 269, 23471–23476 (1994).
Spasojevic, I. et al. Mn porphyrin-based SOD mimic, Mnte-2-Pyp5+, targets mouse heart mitochondria. Free Radic. Biol. Med. 42, 1193–1200 (2007).
Batinic-Haberle, I., Benov, L., Spasojevic, I. & Fridovich, I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 273, 24521–24528 (1998).
Mackensen, G. B. et al. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. J. Neurosci. 21, 4582–4592 (2001).
Vujaskovic, Z. et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic. Biol. Med. 33, 857–863 (2002).
Moeller, B. J. et al. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int. J. Radiat. Oncol. Biol. Phys. 63, 545–552 (2005).
Orrell, R. W. AEOL-10150 (Aeolus). Curr. Opin. Investig. Drugs 7, 70–80 (2006). An overview of AEOL-10150, a manganese porphyrinic antioxidant with neuroprotective actions, entering a Phase I clinical trial.
Sheng, H. et al. Effects of metalloporphyrin catalytic antioxidants in experimental brain ischemia. Free Radic. Biol. Med. 33, 947–961 (2002).
Sheng, H., Spasojevic, I., Warner, D. S. & Batinic-Haberle, I. Mouse spinal cord compression injury is ameliorated by intrathecal cationic manganese(III) porphyrin catalytic antioxidant therapy. Neurosci. Lett. 366, 220–225 (2004).
Crow, J. P., Calingasan, N. Y., Chen, J., Hill, J. L. & Beal, M. F. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann. Neurol. 58, 258–265 (2005).
Shimanovich, R. et al. Mn(II)-texaphyrin as a catalyst for the decomposition of peroxynitrite. J. Am. Chem. Soc. 123, 3613–3614 (2001).
Sharpe, M. A., Ollosson, R., Stewart, V. C. & Clark, J. B. Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem. J. 366, 97–107 (2002).
Schepetkin, I. et al. Decomposition of reactive oxygen species by copper(II) bis(1-pyrazolyl)methane complexes. J. Biol. Inorg. Chem. 11, 499–513 (2006).
Asayama, S., Kawamura, E., Nagaoka, S. & Kawakami, H. Design of manganese porphyrin modified with mitochondrial signal peptide for a new antioxidant. Mol. Pharm. 3, 468–470 (2006).
Szabó, C., Salzman, A. L. & Ischiropoulos, H. Endotoxin triggers the expression of an inducible isoform of NO synthase and the formation of peroxynitrite in the rat aorta in vivo. FEBS Lett. 363, 235–238 (1995).
Szabó, C., Salzman, A. L. & Ischiropoulos, H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia-reperfusion injury. FEBS Lett. 372, 229–232 (1995).
Fukuyama, N. et al. Clinical evidence of peroxynitrite formation in chronic renal failure patients with septic shock. Free Radic. Biol. Med. 22, 771–774 (1997).
Takakura, K., Xiaohong, W., Takeuchi, K., Yasuda, Y. & Fukuda, S. Deactivation of norepinephrine by peroxynitrite as a new pathogenesis in the hypotension of septic shock. Anesthesiology 98, 928–934 (2003).
Delaney, C. A. et al. Sensitivity of human pancreatic islets to peroxynitrite-induced cell dysfunction and death. FEBS Lett. 394, 300–306 (1996).
Suarez-Pinzon, W. L., Szabó, C. & Rabinovitch, A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet β-cells. Diabetes 46, 907–911 (1997).
Suarez-Pinzon, W. L. et al. An inhibitor of inducible NO synthase and scavenger of peroxynitrite prevents diabetes development in NOD mice. J. Autoimmun. 16, 449–455 (2001).
Bottino, R. et al. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes 51, 2561–2567 (2002).
Piganelli, J. D. et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 51, 347–355 (2002).
Olcott, A. P. et al. A salen-manganese catalytic free radical scavenger inhibits type 1 diabetes and islet allograft rejection. Diabetes 53, 2574–2580 (2004).
Del Guerra, S. et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54, 727–735 (2005).
Thuraisingham, R. C., Nott, C. A., Dodd, S. M. & Yaqoob, M. M. Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int. 57, 1968–1972 (2000).
Szabó, C. et al. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation 106, 2680–2686 (2002).
Garcia Soriano, F. et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nature Med. 7, 108–113 (2001).
Devaraj, S. et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55, 774–779 (2006).
Pacher, P., Schulz, R., Liaudet, L. & Szabó, C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol. Sci. 26, 302–310 (2005).
Flesch, M. et al. Effects of endotoxin on human myocardial contractility involvement of NO and peroxynitrite. J. Am. Coll. Cardiol. 33, 1062–1070 (1996).
Lokuta, A. J. et al. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation 111, 988–995 (2005).
Linke, A. et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111, 1763–1770 (2005).
Hunt, M. J. et al. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L239–L245 (2002).
Stewart, V. C. & Heales, S. J. NO-induced mitochondrial dysfunction: implications for neurodegeneration. Free Radic. Biol. Med. 34, 287–303 (2003).
Schulz, J. B., Matthews, R. T., Klockgether, T., Dichgans, J. & Beal, M. F. The role of mitochondrial dysfunction and neuronal NO in animal models of neurodegenerative diseases. Mol. Cell. Biochem. 174, 193–197 (1997).
Torreilles, F., Salman-Tabcheh, S., Guerin, M. & Torreilles, J. Neurodegenerative disorders: the role of peroxynitrite. Brain Res. Brain Res. Rev. 30, 153–163 (1999).
Liu, J. S., Zhao, M. L., Brosnan, C. F. & Lee, S. C. Expression of inducible NO synthase and nitrotyrosine in multiple sclerosis lesions. Am. J. Pathol. 158, 2057–2066 (2001).
Mapp, P. I. et al. Localization of 3-nitrotyrosine to rheumatoid and normal synovium. Arthritis Rheum. 44, 1534–1539 (2001).
Kimura, H. et al. Increased expression of an inducible isoform of NO synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut 42, 180–187 (1998).
Forster, C., Clark, H. B., Ross, M. E. & Iadecola, C. Inducible NO synthase expression in human cerebral infarcts. Acta Neuropathol. 97, 215–220 (1999).
Baker, C. S. et al. Immunocytochemical evidence for inducible NO synthase and cyclooxygenase-2 expression with nitrotyrosine formation in human hibernating myocardium. Basic Res. Cardiol. 97, 409–415 (2002).
Levrand, S. et al. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic. Biol. Med. 41, 886–895 (2006).
Greenacre, S. A. & Ischiropoulos, H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic. Res. 34, 541–581 (2001).
Wu, A. S. et al. Iron porphyrin treatment extends survival in a transgenic animal model of amyotrophic lateral sclerosis. J. Neurochem. 85, 142–150 (2003).
Yamaguchi, Y. et al. Peroxynitrite formation during rat hepatic allograft rejection. Hepatology 29, 777–784 (1999).
Sakurai, M. et al. Quantitative analysis of cardiac 3-L-nitrotyrosine during acute allograft rejection in an experimental heart transplantation. Transplantation 68, 1818–1822 (1999).
Van der Loo, B. et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp Med. 192, 1731–1744 (2000).
Xu, S. et al. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 290, H2220–H2226 (2006).
Seo, A. Y. et al. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid. Redox Signal. 8, 529–538 (2006).
Radovits, T. et al. The peroxynitrite decomposition catalyst FP15 improves ageing-associated cardiac and vascular dysfunction. Mech. Ageing Dev. 128, 173–181 (2007).
Vadseth, C. et al. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J. Biol. Chem. 279, 8820–8826 (2004).
Marcondes, S., Turko, I. V. & Murad, F. Nitration of succinyl-CoA:3-oxoacid CoA-transferase in rats after endotoxin administration. Proc. Natl Acad. Sci. USA 98, 7146–7151 (2001).
Turko, I. V., Marcondes, S. & Murad, F. Diabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-oxoacid CoA-transferase. Am. J. Physiol. Heart Circ. Physiol. 281, H2289–H2294 (2001).
Lees, K. R. et al. Stroke-Acute Ischemic NXY Treatment (SAINT I) Trial Investigators. NXY-059 for acute ischemic stroke. N. Engl. J. Med. 354, 588–600 (2006).
Cable, E. E., Gildemeister, O. S., Pepe, J. A., Lambrecht, R. W. & Bonkovsky, H. L. Mechanism of induction of heme oxygenase by metalloporphyrins in primary chick embryo liver cells: evidence against a stress-mediated response. Mol. Cell. Biochem. 169, 13–20 (1997).
Bowler, R. P. et al. A catalytic antioxidant (AEOL 10150) attenuates expression of inflammatory genes in stroke. Free Radic. Biol. Med. 33, 1141–1152 (2002).
Tumurkhuu, G. et al. MnTBAP, a synthetic metalloporphyrin, inhibits production of tumor necrosis factor-α in lipopolysaccharide-stimulated RAW 264.7 macrophages cells via inhibiting oxidative stress-mediating p38 and SAPK/JNK signaling. FEMS Immunol. Med. Microbiol. 49, 304–311 (2007).
Acknowledgements
We thank V. Valez and D. Vitturi (Universidad de la República, Uruguay) for their contribution to the artwork. We also thank G. Ferrer-Sueta (Universidad de la República, Uruguay) for useful discussions. This work was supported by the NIH R01 GM060915 grant and the Oscar Asboth Project Grant from the National Office of Research and Technology, Budapest, Hungary to C.S.; HL54926, AG13966, ES013508 NIEHS Center of Excellence in Environmental Toxicology grants to H.I.; and The Howard Hughes Medical Institute and the International Centre of Genetic Engineering and Biotechnology grant to R.R. H.I. is the Gisela and Dennis Alter Chair in Pediatric Neonatology at the Children's Hospital of Philadelphia. R.R. is a Howard Hughes International Research Scholar.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
C.S. is a founder, stockholder and consultant to Inotek Pharmaceuticals Corporation, a pharmaceutical firm that is involved in the development of peroxynitrite decomposition catalysts.
Supplementary information
Supplementary information S1 (box)
Exposure of biological systems to peroxynitrite (PDF 109 kb)
Supplementary information S2 (box)
Practical aspects of working with peroxynitrite (PDF 107 kb)
Supplementary information S3 (box)
Peroxynitrite – a mediator of cell death (PDF 102 kb)
Related links
Glossary
- Nitric oxide
-
The product of nitric oxide (•NO) synthases, a family of proteins that catalyze the oxidation of the guanidine group of L-arginine to citrulline and •NO.
- Superoxide
-
The product of the one-electron reduction of molecular oxygen.
- Transition metal centres
-
Complexes of biomolecules with transition metals such as iron, manganese or copper that can participate in redox chemistry.
- Radical–radical termination
-
A reaction between two free radicals, which leads to a non-free radical adduct as a reaction product and therefore stops radical propagation reactions.
- Homolytic fission
-
Rupture of a covalent bond in a molecule, in which the two resulting products keep one of the bond electrons (for example, A:B → A. + B.).
- Tetrahydrobiopterin
-
A cofactor that carries electrons for redox reactions. It serves as a cofactor for nitric oxide synthase.
- Mitochondrial electron-transport chain
-
A series of redox carrier proteins in the inner mitochondrial membrane that enable the flow of electrons from respiratory substrates to molecular oxygen. The potential energy inherent in the electron gradient is used to drive the synthesis of ATP when protons flow back across the membrane through another enzyme complex, ATP synthase.
- Cytochrome c
-
An evolutionally highly conserved small 12,000 daltons haem protein present in the mitochondrial intermembrane space. It participates in mitochondrial electron transport and can also serve as a pro-apoptotic signal if released into the cytosol.
- Mn superoxide dismutase
-
A key manganese-containing mitochondrial antioxidant enzyme that catalyzes superoxide radical dismutation.
- Poly(ADP-ribose) polymerase
-
(PARP). Several reactive oxygen and nitrogen species can trigger DNA strand breakage, which then activates the nuclear enzyme PARP. Rapid activation of the enzyme depletes the intracellular concentration of its substrate, NAD, thus slowing the rate of glycolysis, electron transport and subsequently ATP formation. PARP plays a physiological role in the cells to facilitate DNA repair and to maintain genomic integrity.
- Permeability transition pore
-
A mitochondrial multiprote in structure that once activated serves for the release of pro-apoptotic factors into the cytosol.
- Sepsis
-
A serious systemic inflammatory disease condition associated with fever, elevated white blood cell count, raised heart rate and increased breathing rate. Severe sepsis can be also associated with multiple organ failure and circulatory collapse.
- Porphyrin
-
A macrocyclic organic molecule consisting of four pyrrole rings that participates in the structure of haem proteins such as haemoglobin and cytochromes. It can tightly bind iron and manganese.
- Catecholamines
-
A group of endogenous amines (adrenaline, noradrenaline and dopamine) derived from catechol that have important physiological effects as neurotransmitters and hormones. Some of their effects include increases in heart rate, blood pressure and blood glucose levels.
- Chelator
-
A molecule that has the capacity of tight metal binding (complexation).
- Dismutation
-
A chemical reaction between two identical molecules to produce two different products (for example, superoxide dismutation to molecular oxygen and hydrogen peroxide).
- Cage return reaction
-
In the course of a chemical reaction, the reaction between two transient products before they diffuse out of the 'solvent cage'.
- Atropoisomerism
-
A type of stereoisomerism that may arise in systems in which free rotation around a single covalent bond is impeded sufficiently so as to allow different stereoisomers to be isolated.
Rights and permissions
About this article
Cite this article
Szabó, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6, 662–680 (2007). https://doi.org/10.1038/nrd2222
Issue Date:
DOI: https://doi.org/10.1038/nrd2222
This article is cited by
-
N,N-dimethylacetamide targets neuroinflammation in Alzheimer’s disease in in-vitro and ex-vivo models
Scientific Reports (2023)
-
Acetovanillone augmented the cardioprotective effect of carvedilol against cadmium-induced heart injury via suppression of oxidative stress and inflammation signaling pathways
Scientific Reports (2023)
-
Nitration of chemokine CXCL8 acts as a natural mechanism to limit acute inflammation
Cellular and Molecular Life Sciences (2023)
-
Nitration of Tyrosine and Tyrosyl Residues in Myoglobin by the Action of Visible Light in the Presence of Riboflavin and Nitrite**
Journal of Applied Spectroscopy (2023)
-
Pollutants in aquatic system: a frontier perspective of emerging threat and strategies to solve the crisis for safe drinking water
Environmental Science and Pollution Research (2023)