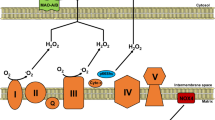
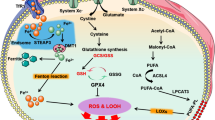
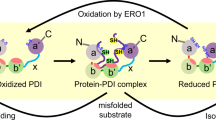
Abstract
Reactive oxygen species (ROS)-dependent production of ROS underlies sustained oxidative stress, which has been implicated in the pathogenesis of cardiovascular diseases such as hypertension, aortic aneurysm, hypercholesterolaemia, atherosclerosis, diabetic vascular complications, cardiac ischaemia–reperfusion injury, myocardial infarction, heart failure and cardiac arrhythmias. Interactions between different oxidases or oxidase systems have been intensively investigated for their roles in inducing sustained oxidative stress. In this Review, we discuss the latest data on the pathobiology of each oxidase component, the complex crosstalk between different oxidase components and the consequences of this crosstalk in mediating cardiovascular disease processes, focusing on the central role of particular NADPH oxidase (NOX) isoforms that are activated in specific cardiovascular diseases. An improved understanding of these mechanisms might facilitate the development of novel therapeutic agents targeting these oxidase systems and their interactions, which could be effective in the prevention and treatment of cardiovascular disorders.
Key points
-
Activation of NADPH oxidase (NOX) has a critical role in the pathogenesis of cardiovascular diseases.
-
Activation of NOX induces activation of downstream secondary oxidase systems, including uncoupled endothelial nitric oxide synthase, dysfunctional mitochondria and xanthine oxidase.
-
Crosstalk between oxidases or oxidase systems sustains oxidative stress to mediate the development of cardiovascular diseases.
-
Targeting NOXs as well as interactions between NOXs and secondary oxidase systems might be a novel therapeutic strategy for the prevention and treatment of cardiovascular diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cai, H. & Harrison, D. G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–844 (2000).
Brown, D. I. & Griendling, K. K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 116, 531–549 (2015).
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L. & Sollott, S. J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192, 1001–1014 (2000).
Zinkevich, N. S. & Gutterman, D. D. ROS-induced ROS release in vascular biology: redox-redox signaling. Am. J. Physiol. Heart Circ. Physiol. 301, H647–H653 (2011).
Cai, H., Griendling, K. K. & Harrison, D. G. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 24, 471–478 (2003).
Cai, H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 68, 26–36 (2005).
Cai, H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ. Res. 96, 818–822 (2005).
Youn, J. Y., Siu, K. L., Li, Q., Harrison, D. G. & Cai, H. in Systems biology of free radicals and antioxidants (ed. Laher, I.) 849–876 (Springer, Berlin, Heidelberg, 2014).
Wever, R. M., van Dam, T., van Rijn, H. J., de Groot, F. & Rabelink, T. J. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 237, 340–344 (1997).
Vasquez-Vivar, J. et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl Acad. Sci. USA 95, 9220–9225 (1998).
Xia, Y., Tsai, A. L., Berka, V. & Zweier, J. L. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J. Biol. Chem. 273, 25804–25808 (1998).
Laursen, J. B. et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103, 1282–1288 (2001).
Landmesser, U. et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 111, 1201–1209 (2003).
Alp, N. J., McAteer, M. A., Khoo, J., Choudhury, R. P. & Channon, K. M. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler. Thromb. Vasc. Biol. 24, 445–450 (2004).
Chalupsky, K. & Cai, H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 102, 9056–9061 (2005).
Takimoto, E. et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Invest. 115, 1221–1231 (2005).
Oak, J. H. & Cai, H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes 56, 118–126 (2007).
Takaya, T. et al. A specific role for eNOS-derived reactive oxygen species in atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol. 27, 1632–1637 (2007).
Hattori, Y. et al. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler. Thromb. Vasc. Biol. 27, 865–870 (2007).
Du, Y. H., Guan, Y. Y., Alp, N. J., Channon, K. M. & Chen, A. F. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117, 1045–1054 (2008).
Wang, S. et al. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension 52, 484–490 (2008).
Gao, L. et al. Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol. 297, H331–H339 (2009).
Gao, L., Chalupsky, K., Stefani, E. & Cai, H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J. Mol. Cell. Cardiol. 47, 752–760 (2009).
Crabtree, M. J. & Channon, K. M. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25, 81–88 (2011).
Li, L., Chen, W., Rezvan, A., Jo, H. & Harrison, D. G. Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arterioscler. Thromb. Vasc. Biol. 31, 1547–1554 (2011).
Youn, J. Y., Gao, L. & Cai, H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 55, 2069–2079 (2012).
Gao, L. et al. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: treatment with folic acid. Hypertension 59, 158–166 (2012).
Youn, J. Y. et al. Endothelium-specific sepiapterin reductase deficiency in DOCA-salt hypertension. Am. J. Physiol. Heart Circ. Physiol. 302, H2243–H2249 (2012).
Siu, K. L., Miao, X. N. & Cai, H. Recoupling of eNOS with folic acid prevents abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E null mice. PLOS ONE 9, e88899 (2014).
Siu, K. L. & Cai, H. Circulating tetrahydrobiopterin as a novel biomarker for abdominal aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol. 307, H1559–H1564 (2014).
Siu, K. L., Lotz, C., Ping, P. & Cai, H. Netrin-1 abrogates ischemia/reperfusion-induced cardiac mitochondrial dysfunction via nitric oxide-dependent attenuation of NOX4 activation and recoupling of NOS. J. Mol. Cell. Cardiol. 78, 174–185 (2015).
Miao, X. N., Siu, K. L. & Cai, H. Nifedipine attenuation of abdominal aortic aneurysm in hypertensive and non-hypertensive mice: mechanisms and implications. J. Mol. Cell. Cardiol. 87, 152–159 (2015).
Li, Q., Youn, J. Y. & Cai, H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypertens. 33, 1128–1136 (2015).
Siu, K. L. et al. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol. 11, 118–125 (2017).
Youn, J. Y., Zhou, J. & Cai, H. Bone morphogenic protein 4 mediates NOX1-dependent eNOS uncoupling, endothelial dysfunction, and COX2 induction in type 2 diabetes mellitus. Mol. Endocrinol. 29, 1123–1133 (2015).
Li, H. et al. Novel treatment of hypertension by specifically targeting E2F for restoration of endothelial dihydrofolate reductase and eNOS function under oxidative stress. Hypertension 73, 179–189 (2019).
Li, Q. et al. Knockout of dihydrofolate reductase in mice induces hypertension and abdominal aortic aneurysm via mitochondrial dysfunction. Redox Biol. 24, 101185 (2019).
Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta 1797, 897–906 (2010).
Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 51, 1289–1301 (2011).
Daiber, A. et al. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 174, 1670–1689 (2017).
Doughan, A. K., Harrison, D. G. & Dikalov, S. I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 102, 488–496 (2008).
Zhang, D. X. et al. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ. Res. 89, 1177–1183 (2001).
Loperena, R. & Harrison, D. G. Oxidative stress and hypertensive diseases. Med. Clin. North Am. 101, 169–193 (2017).
Kigawa, Y. et al. NADPH oxidase deficiency exacerbates angiotensin II-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 34, 2413–2420 (2014).
Forstermann, U., Xia, N. & Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120, 713–735 (2017).
Amanso, A. M. & Griendling, K. K. Differential roles of NADPH oxidases in vascular physiology and pathophysiology. Front. Biosci. 4, 1044–1064 (2012).
Konior, A., Schramm, A., Czesnikiewicz-Guzik, M. & Guzik, T. J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 20, 2794–2814 (2014).
Matsushima, S., Tsutsui, H. & Sadoshima, J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med. 24, 202–205 (2014).
Kahles, T. & Brandes, R. P. NADPH oxidases as therapeutic targets in ischemic stroke. Cell. Mol. Life Sci. 69, 2345–2363 (2012).
Carbone, F. et al. Pathophysiology and treatments of oxidative injury in ischemic stroke: focus on the phagocytic nadph oxidase 2. Antioxid. Redox Signal. 23, 460–489 (2015).
Zhang, M., Perino, A., Ghigo, A., Hirsch, E. & Shah, A. M. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid. Redox Signal. 18, 1024–1041 (2013).
Sag, C. M., Santos, C. X. & Shah, A. M. Redox regulation of cardiac hypertrophy. J. Mol. Cell. Cardiol. 73, 103–111 (2014).
Youn, J. Y. et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J. Mol. Cell. Cardiol. 62, 72–79 (2013).
Guzik, T. J. & Harrison, D. G. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov. Today 11, 524–533 (2006).
Briones, A. M. & Touyz, R. M. Oxidative stress and hypertension: current concepts. Curr. Hypertens. Rep. 12, 135–142 (2010).
Wang, H. D. et al. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 88, 947–953 (2001).
Barry-Lane, P. A. et al. p47phox is required for atherosclerotic lesion progression in ApoE-/- mice. J. Clin. Invest. 108, 1513–1522 (2001).
Matsushima, S. et al. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1α and upregulation of peroxisome proliferator-activated receptor-α. Circ. Res. 112, 1135–1149 (2013).
Kuroda, J. et al. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl Acad. Sci. USA 107, 15565–15570 (2010).
Zhang, Y. et al. NADPH oxidase 4 induces cardiac arrhythmic phenotype in zebrafish. J. Biol. Chem. 289, 23200–23208 (2014).
Iyer, G. Y., Islam, M. F. & Quastel, J. H. Biochemical aspects of phagocytosis. Nature 192, 535–541 (1961).
Rossi, F. & Zatti, M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia 20, 21–23 (1964).
Segal, A. W. & Jones, O. T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 276, 515–517 (1978).
Segal, A. W., Jones, O. T., Webster, D. & Allison, A. C. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet 2, 446–449 (1978).
Royer-Pokora, B. et al. Cloning the gene for an inherited human disorder–chronic granulomatous disease–on the basis of its chromosomal location. Nature 322, 32–38 (1986).
Dinauer, M. C., Orkin, S. H., Brown, R., Jesaitis, A. J. & Parkos, C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 327, 717–720 (1987).
Nunoi, H., Rotrosen, D., Gallin, J. I. & Malech, H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science 242, 1298–1301 (1988).
Volpp, B. D., Nauseef, W. M. & Clark, R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 242, 1295–1297 (1988).
Abo, A. et al. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353, 668–670 (1991).
Knaus, U. G., Heyworth, P. G., Evans, T., Curnutte, J. T. & Bokoch, G. M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 254, 1512–1515 (1991).
Wientjes, F. B., Hsuan, J. J., Totty, N. F. & Segal, A. W. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem. J. 296, 557–561 (1993).
Griendling, K. K., Minieri, C. A., Ollerenshaw, J. D. & Alexander, R. W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 74, 1141–1148 (1994).
Suh, Y. A. et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401, 79–82 (1999).
Banfi, B. et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287, 138–142 (2000).
Kikuchi, H., Hikage, M., Miyashita, H. & Fukumoto, M. NADPH oxidase subunit, gp91phox homologue, preferentially expressed in human colon epithelial cells. Gene 254, 237–243 (2000).
Geiszt, M., Kopp, J. B., Varnai, P. & Leto, T. L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl Acad. Sci. USA 97, 8010–8014 (2000).
De Deken, X. et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 275, 23227–23233 (2000).
Edens, W. A. et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 154, 879–891 (2001).
Banfi, B. et al. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276, 37594–37601 (2001).
Cheng, G., Cao, Z., Xu, X., van Meir, E. G. & Lambeth, J. D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269, 131–140 (2001).
Banfi, B., Clark, R. A., Steger, K. & Krause, K. H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278, 3510–3513 (2003).
Geiszt, M., Lekstrom, K., Witta, J. & Leto, T. L. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 278, 20006–20012 (2003).
Takeya, R. et al. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 278, 25234–25246 (2003).
Grasberger, H. & Refetoff, S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem. 281, 18269–18272 (2006).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Gimenez, M., Schickling, B. M., Lopes, L. R. & Miller, F. J. Jr. Nox1 in cardiovascular diseases: regulation and pathophysiology. Clin. Sci. 130, 151–165 (2016).
Dinauer, M. C., Curnutte, J. T., Rosen, H. & Orkin, S. H. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J. Clin. Invest. 84, 2012–2016 (1989).
Ueno, N., Takeya, R., Miyano, K., Kikuchi, H. & Sumimoto, H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J. Biol. Chem. 280, 23328–23339 (2005).
Ago, T. et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 106, 1253–1264 (2010).
Oda, T. et al. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J. Biol. Chem. 285, 1435–1445 (2010).
Magnani, F. et al. Crystal structures and atomic model of NADPH oxidase. Proc. Natl Acad. Sci. USA 114, 6764–6769 (2017).
Brandes, R. P., Weissmann, N. & Schroder, K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic. Biol. Med. 76, 208–226 (2014).
Van Buul, J. D., Fernandez-Borja, M., Anthony, E. C. & Hordijk, P. L. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal. 7, 308–317 (2005).
Mizuno, T. et al. Regulation of the superoxide-generating NADPH oxidase by a small GTP-binding protein and its stimulatory and inhibitory GDP/GTP exchange proteins. J. Biol. Chem. 267, 10215–10218 (1992).
Kwong, C. H., Malech, H. L., Rotrosen, D. & Leto, T. L. Regulation of the human neutrophil NADPH oxidase by rho-related G-proteins. Biochemistry 32, 5711–5717 (1993).
Kim, C. & Dinauer, M. C. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J. Immunol. 166, 1223–1232 (2001).
Fontayne, A., Dang, P. M., Gougerot-Pocidalo, M. A. & El-Benna, J. Phosphorylation of p47phox sites by PKC α, βII, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41, 7743–7750 (2002).
Kitada, M. et al. Translocation of glomerular p47phox and p67phox by protein kinase C-β activation is required for oxidative stress in diabetic nephropathy. Diabetes 52, 2603–2614 (2003).
Schulz, E., Wenzel, P., Munzel, T. & Daiber, A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 20, 308–324 (2014).
Groemping, Y., Lapouge, K., Smerdon, S. J. & Rittinger, K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113, 343–355 (2003).
Seshiah, P. N. et al. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ. Res. 91, 406–413 (2002).
Altenhofer, S., Radermacher, K. A., Kleikers, P. W., Wingler, K. & Schmidt, H. H. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 23, 406–427 (2015).
Sahoo, S., Meijles, D. N. & Pagano, P. J. NADPH oxidases: key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 130, 317–335 (2016).
Lyle, A. N. et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 105, 249–259 (2009).
Banfi, B. et al. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J. Biol. Chem. 279, 18583–18591 (2004).
Jha, J. C., Watson, A. M. D., Mathew, G., de Vos, L. C. & Jandeleit-Dahm, K. The emerging role of NADPH oxidase NOX5 in vascular disease. Clin. Sci. 131, 981–990 (2017).
Chen, F., Yin, C., Dimitropoulou, C. & Fulton, D. J. Cloning, characteristics, and functional analysis of rabbit NADPH oxidase 5. Front. Physiol. 7, 284 (2016).
BelAiba, R. S. et al. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 42, 446–459 (2007).
Chen, F., Wang, Y., Barman, S. & Fulton, D. J. Enzymatic regulation and functional relevance of NOX5. Curr. Pharm. Des. 21, 5999–6008 (2015).
Tirone, F. & Cox, J. A. NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett. 581, 1202–1208 (2007).
Chen, F. et al. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLOS ONE 9, e88405 (2014).
Pandey, D., Gratton, J. P., Rafikov, R., Black, S. M. & Fulton, D. J. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol. Pharmacol. 80, 407–415 (2011).
Pandey, D. & Fulton, D. J. Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am. J. Physiol. Heart Circ. Physiol. 300, H1336–H1344 (2011).
Montezano, A. C. et al. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr. Opin. Nephrol. Hypertens. 24, 425–433 (2015).
Lambeth, J. D., Kawahara, T. & Diebold, B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 43, 319–331 (2007).
Lassegue, B., San Martin, A. & Griendling, K. K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 110, 1364–1390 (2012).
Brandes, R. P. & Schroder, K. Differential vascular functions of Nox family NADPH oxidases. Curr. Opin. Lipidol. 19, 513–518 (2008).
Ago, T. et al. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke 36, 1040–1046 (2005).
Gorlach, A. et al. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 87, 26–32 (2000).
Guzik, T. J. et al. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 52, 1803–1809 (2008).
Ellmark, S. H., Dusting, G. J., Fui, M. N., Guzzo-Pernell, N. & Drummond, G. R. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 65, 495–504 (2005).
Matsuno, K. et al. NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free Radic. Biol. Med. 53, 1718–1728 (2012).
Morawietz, H. & Bornstein, S. R. Leptin, endothelin, NADPH oxidase, and heart failure. Hypertension 47, e20 (2006).
Heymes, C. et al. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 41, 2164–2171 (2003).
Krijnen, P. A. et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J. Clin. Pathol. 56, 194–199 (2003).
Hahn, N. E. et al. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am. J. Pathol. 180, 2222–2229 (2012).
Chen, K., Kirber, M. T., Xiao, H., Yang, Y. & Keaney, J. F. Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 181, 1129–1139 (2008).
Wu, R. F., Ma, Z., Liu, Z. & Terada, L. S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 30, 3553–3568 (2010).
Hilenski, L. L., Clempus, R. E., Quinn, M. T., Lambeth, J. D. & Griendling, K. K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 24, 677–683 (2004).
Clempus, R. E. et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 27, 42–48 (2007).
Perrotta, I., Sciangula, A., Perrotta, E., Donato, G. & Cassese, M. Ultrastructural analysis and electron microscopic localization of Nox4 in healthy and atherosclerotic human aorta. Ultrastruct. Pathol. 35, 1–6 (2011).
Camargo, L. L. et al. Vascular NOX (NADPH oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension 72, 235–246 (2018).
Ago, T. et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109, 227–233 (2004).
Matsushima, S. et al. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 112, 651–663 (2013).
Dikalov, S. I. et al. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 45, 1340–1351 (2008).
Helmcke, I., Heumuller, S., Tikkanen, R., Schroder, K. & Brandes, R. P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid. Redox. Signal. 11, 1279–1287 (2009).
Takac, I. et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 286, 13304–13313 (2011).
Cai, H., Dikalov, S., Griendling, K. K. & Harrison, D. G. Detection of reactive oxygen species and nitric oxide in vascular cells and tissues: comparison of sensitivity and specificity. Methods Mol. Med. 139, 293–311 (2007).
Schulz, E., Jansen, T., Wenzel, P., Daiber, A. & Munzel, T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 10, 1115–1126 (2008).
Forstermann, U. & Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 164, 213–223 (2011).
Thony, B., Auerbach, G. & Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347, 1–16 (2000).
Hink, U. et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 88, E14–E22 (2001).
Faria, A. M., Papadimitriou, A., Silva, K. C., Lopes de Faria, J. M. & Lopes de Faria, J. B. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 61, 1838–1847 (2012).
Moens, A. L. et al. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation 117, 1810–1819 (2008).
Moens, A. L. et al. Bi-modal dose-dependent cardiac response to tetrahydrobiopterin in pressure-overload induced hypertrophy and heart failure. J. Mol. Cell. Cardiol. 51, 564–569 (2011).
Zheng, J. S. et al. Gene transfer of human guanosine 5’-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation 108, 1238–1245 (2003).
Raman, C. S. et al. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell 95, 939–950 (1998).
Li, H. et al. Crystal structures of zinc-free and -bound heme domain of human inducible nitric-oxide synthase. Implications for dimer stability and comparison with endothelial nitric-oxide synthase. J. Biol. Chem. 274, 21276–21284 (1999).
Hemmens, B., Goessler, W., Schmidt, K. & Mayer, B. Role of bound zinc in dimer stabilization but not enzyme activity of neuronal nitric-oxide synthase. J. Biol. Chem. 275, 35786–35791 (2000).
Zou, M. H., Shi, C. & Cohen, R. A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest. 109, 817–826 (2002).
Xu, J., Xie, Z., Reece, R., Pimental, D. & Zou, M. H. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 26, 2688–2695 (2006).
Chen, C. A. et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468, 1115–1118 (2010).
Knorr, M. et al. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler. Thromb. Vasc. Biol. 31, 2223–2231 (2011).
Oelze, M. et al. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur. Heart J. 34, 3206–3216 (2013).
Schuhmacher, S. et al. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes 60, 2608–2616 (2011).
Heinzel, B., John, M., Klatt, P., Bohme, E. & Mayer, B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem. J. 281, 627–630 (1992).
Pou, S., Pou, W. S., Bredt, D. S., Snyder, S. H. & Rosen, G. M. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 267, 24173–24176 (1992).
Xia, Y., Dawson, V. L., Dawson, T. M., Snyder, S. H. & Zweier, J. L. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc. Natl Acad. Sci. USA 93, 6770–6774 (1996).
Loughran, P. A. et al. Monomeric inducible nitric oxide synthase localizes to peroxisomes in hepatocytes. Proc. Natl Acad. Sci. USA 102, 13837–13842 (2005).
Chance, B., Sies, H. & Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605 (1979).
Trono, D., Laus, M. N., Soccio, M., Alfarano, M. & Pastore, D. Modulation of potassium channel activity in the balance of ROS and ATP production by durum wheat mitochondria — an amazing defense tool against hyperosmotic stress. Front. Plant Sci. 6, 1072 (2015).
Queliconi, B. B., Wojtovich, A. P., Nadtochiy, S. M., Kowaltowski, A. J. & Brookes, P. S. Redox regulation of the mitochondrial KATP channel in cardioprotection. Biochim. Biophys. Acta 1813, 1309–1315 (2011).
Oldenburg, O., Cohen, M. V., Yellon, D. M. & Downey, J. M. Mitochondrial KATP channels: role in cardioprotection. Cardiovasc. Res. 55, 429–437 (2002).
Malinska, D., Mirandola, S. R. & Kunz, W. S. Mitochondrial potassium channels and reactive oxygen species. FEBS Lett. 584, 2043–2048 (2010).
Brandes, R. P. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension 45, 847–848 (2005).
Kimura, S. et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension 45, 438–444 (2005).
Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005).
Jastroch, M., Divakaruni, A. S., Mookerjee, S., Treberg, J. R. & Brand, M. D. Mitochondrial proton and electron leaks. Essays Biochem. 47, 53–67 (2010).
Madamanchi, N. R. & Runge, M. S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 100, 460–473 (2007).
Cadenas, E., Boveris, A., Ragan, C. I. & Stoppani, A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome C reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 180, 248–257 (1977).
Han, D., Williams, E. & Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 353, 411–416 (2001).
Ago, T., Kuroda, J., Kamouchi, M., Sadoshima, J. & Kitazono, T. Pathophysiological roles of NADPH oxidase/NOX family proteins in the vascular system. Review and perspective. Circ. J. 75, 1791–1800 (2011).
Graham, D. et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54, 322–328 (2009).
Ballinger, S. W. et al. Mitochondrial integrity and function in atherogenesis. Circulation 106, 544–549 (2002).
Chen, J., Stimpson, S. E., Fernandez-Bueno, G. A. & Mathews, C. E. Mitochondrial reactive oxygen species and type 1 diabetes. Antioxid. Redox Signal. 29, 1361–1372 (2018).
Anderson, E. J. et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119, 573–581 (2009).
Ide, T. et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 85, 357–363 (1999).
Dai, D. F. et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Gαq overexpression-induced heart failure. Circ. Res. 108, 837–846 (2011).
Munzel, T., Gori, T., Keaney, J. F. Jr, Maack, C. & Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 36, 2555–2564 (2015).
Escribano-Lopez, I. et al. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 10, 200–205 (2016).
Ohashi, M., Runge, M. S., Faraci, F. M. & Heistad, D. D. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 26, 2331–2336 (2006).
Nishikawa, T. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790 (2000).
Dai, D. F. et al. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J. Am. Coll. Cardiol. 58, 73–82 (2011).
Hille, R. & Nishino, T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 9, 995–1003 (1995).
Christen, S., Bifrare, Y. D., Siegenthaler, C., Leib, S. L. & Tauber, M. G. Marked elevation in cortical urate and xanthine oxidoreductase activity in experimental bacterial meningitis. Brain Res. 900, 244–251 (2001).
Nagler, R. M., Klein, I., Zarzhevsky, N., Drigues, N. & Reznick, A. Z. Characterization of the differentiated antioxidant profile of human saliva. Free Radic. Biol. Med. 32, 268–277 (2002).
Nakazono, K. et al. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl Acad. Sci. USA 88, 10045–10048 (1991).
Suzuki, H. et al. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl Acad. Sci. USA 95, 4754–4759 (1998).
Swei, A., Lacy, F., Delano, F. A., Parks, D. A. & Schmid-Schonbein, G. W. A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirculation 6, 179–187 (1999).
Montor, S. G., Thoolen, M. J., Mackin, W. M. & Timmermans, P. B. Effect of azapropazone and allopurinol on myocardial infarct size in rats. Eur. J. Pharmacol. 140, 203–207 (1987).
Li, G. R. & Ferrier, G. R. Effects of allopurinol on reperfusion arrhythmias in isolated ventricles. Am. J. Physiol. 263, H341–H348 (1992).
Stull, L. B., Leppo, M. K., Szweda, L., Gao, W. D. & Marban, E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ. Res. 95, 1005–1011 (2004).
Engberding, N. et al. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation 110, 2175–2179 (2004).
Segal, M. S. et al. The effect of the addition of allopurinol on blood pressure control in African Americans treated with a thiazide-like diuretic. J. Am. Soc. Hypertens. 9, 610–619.e1 (2015).
Hare, J. M. et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 51, 2301–2309 (2008).
Alem, M. M., Alshehri, A. M., Cahusac, P. M. & Walters, M. R. Effect of xanthine oxidase inhibition on arterial stiffness in patients with chronic heart failure. Clin. Med. Insights Cardiol. 12, 1179546818779584 (2018).
Borghi, C. et al. Effects of the concomitant administration of xanthine oxidase inhibitors with zofenopril or other ACE-inhibitors in post-myocardial infarction patients: a meta-analysis of individual data of four randomized, double-blind, prospective studies. BMC Cardiovasc. Disord. 18, 112 (2018).
Duda, M., Konior, A., Klemenska, E. & Beresewicz, A. Preconditioning protects endothelium by preventing ET-1-induced activation of NADPH oxidase and xanthine oxidase in post-ischemic heart. J. Mol. Cell. Cardiol. 42, 400–410 (2007).
Zhao, Q., Zhang, J. & Wang, H. PGC-1α overexpression suppresses blood pressure elevation in DOCA-salt hypertensive mice. Biosci. Rep. 35, e00217 (2015).
Callera, G. E., Tostes, R. C., Yogi, A., Montezano, A. C. & Touyz, R. M. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin. Sci. 110, 243–253 (2006).
Pain, T. et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ. Res. 87, 460–466 (2000).
Lassegue, B. & Griendling, K. K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 30, 653–661 (2010).
Dikalova, A. E. et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 107, 106–116 (2010).
Rubbo, H. et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 269, 26066–26075 (1994).
Ebadi, M. & Sharma, S. K. Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid. Redox Signal. 5, 319–335 (2003).
Ceylan-Isik, A. F. et al. Metallothionein abrogates GTP cyclohydrolase I inhibition-induced cardiac contractile and morphological defects: role of mitochondrial biogenesis. Hypertension 53, 1023–1031 (2009).
Watts, G. F. et al. Coenzyme Q10 improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia 45, 420–426 (2002).
Chew, G. T. & Watts, G. F. Coenzyme Q10 and diabetic endotheliopathy: oxidative stress and the ‘recoupling hypothesis’. QJM 97, 537–548 (2004).
Vergeade, A. et al. Xanthine oxidase contributes to mitochondrial ROS generation in an experimental model of cocaine-induced diastolic dysfunction. J. Cardiovasc. Pharmacol. 60, 538–543 (2012).
Gladden, J. D. et al. Novel insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free Radic. Biol. Med. 51, 1975–1984 (2011).
Rajagopalan, S. et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 97, 1916–1923 (1996).
Fukui, T. et al. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 80, 45–51 (1997).
Beswick, R. A., Dorrance, A. M., Leite, R. & Webb, R. C. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38, 1107–1111 (2001).
Wu, R., Millette, E., Wu, L. & de Champlain, J. Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and desoxycorticosterone acetate-salt hypertensive rats. J. Hypertens. 19, 741–748 (2001).
Bauersachs, J. et al. Hydralazine prevents endothelial dysfunction, but not the increase in superoxide production in nitric oxide-deficient hypertension. Eur. J. Pharmacol. 362, 77–81 (1998).
Kobori, H. & Nishiyama, A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem. Biophys. Res. Commun. 315, 746–750 (2004).
Zalba, G. et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 35, 1055–1061 (2000).
Delles, C., Miller, W. H. & Dominiczak, A. F. Targeting reactive oxygen species in hypertension. Antioxid. Redox Signal. 10, 1061–1077 (2008).
Sedeek, M., Hebert, R. L., Kennedy, C. R., Burns, K. D. & Touyz, R. M. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr. Opin. Nephrol. Hypertens. 18, 122–127 (2009).
Takac, I., Schroder, K. & Brandes, R. P. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr. Hypertens. Rep. 14, 70–78 (2012).
Higashi, M. et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ. Res. 93, 767–775 (2003).
Matsuno, K. et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112, 2677–2685 (2005).
Wingler, K. et al. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic. Biol. Med. 31, 1456–1464 (2001).
Zhao, Q., Zhang, J. & Wang, H. PGC-1α limits angiotensin II-induced rat vascular smooth muscle cells proliferation via attenuating NOX1-mediated generation of reactive oxygen species. Biosci. Rep. 35, e00252 (2015).
Liang, G. Z. et al. ClC-3 promotes angiotensin II-induced reactive oxygen species production in endothelial cells by facilitating Nox2 NADPH oxidase complex formation. Acta Pharmacol. Sin. 39, 1725–1734 (2018).
Yamagishi, S., Nakamura, K., Ueda, S., Kato, S. & Imaizumi, T. Pigment epithelium-derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel anti-oxidative mechanism of PEDF. Cell Tissue Res. 320, 437–445 (2005).
Montezano, A. C. et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ. Res. 106, 1363–1373 (2010).
Touyz, R. M., Yao, G. & Schiffrin, E. L. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 23, 981–987 (2003).
Touyz, R. M. & Schiffrin, E. L. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 34, 976–982 (1999).
Touyz, R. M. & Schiffrin, E. L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 52, 639–672 (2000).
Garrido, A. M. & Griendling, K. K. NADPH oxidases and angiotensin II receptor signaling. Mol. Cell. Endocrinol. 302, 148–158 (2009).
Nguyen Dinh Cat, A., Montezano, A. C., Burger, D. & Touyz, R. M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 19, 1110–1120 (2013).
Gavazzi, G. et al. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 580, 497–504 (2006).
Weber, D. S. et al. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 288, H37–H42 (2005).
Dikalova, A. et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112, 2668–2676 (2005).
Bendall, J. K. et al. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ. Res. 100, 1016–1025 (2007).
Murdoch, C. E. et al. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res. Cardiol. 106, 527–538 (2011).
Bouabout, G. et al. Nox4 genetic inhibition in experimental hypertension and metabolic syndrome. Arch. Cardiovasc. Dis. 111, 41–52 (2018).
Schroder, K. et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 110, 1217–1225 (2012).
Zhao, Q. D. et al. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways. Circulation 131, 643–655 (2015).
Ray, R. et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 31, 1368–1376 (2011).
Laude, K. et al. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am. J. Physiol. Heart Circ. Physiol. 288, H7–H12 (2005).
Langbein, H. et al. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 37, 1753–1761 (2016).
Drummond, G. R. & Sobey, C. G. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol. Metab. 25, 452–463 (2014).
Montezano, A.C. et al. NADPH oxidase 5 is a pro-contractile nox isoform and a point of cross-talk for calcium and redox signaling-implications in vascular function. J. Am. Heart Assoc. 7, e009388 (2018).
Jha, J. C. et al. NADPH oxidase NOX5 accelerates renal injury in diabetic nephropathy. Diabetes 66, 2691–2703 (2017).
Casas, A. I. et al. Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J. Clin. Invest. 130, 1772–1778 (2019).
Holterman, C. E. et al. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J. Am. Soc. Nephrol. 25, 784–797 (2014).
Cowley, A. W. Jr. et al. Evidence of the importance of NOX4 in production of hypertension in Dahl salt-sensitive rats. Hypertension 67, 440–450 (2016).
Kroller-Schon, S. et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species — studies in white blood cells and in animal models. Antioxid. Redox Signal. 20, 247–266 (2014).
Miller, F. J. Jr. et al. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler. Thromb. Vasc. Biol. 22, 560–565 (2002).
Guzik, B. et al. Mechanisms of oxidative stress in human aortic aneurysms — association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 168, 2389–2396 (2013).
McCormick, M. L., Gavrila, D. & Weintraub, N. L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 27, 461–469 (2007).
Streeter, J., Thiel, W., Brieger, K. & Miller, F. J. Opportunity NOX: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc. Ther. 31, 125–137 (2013).
Aviram, M., Rosenblat, M., Etzioni, A. & Levy, R. Activation of NADPH oxidase required for macrophage-mediated oxidation of low-density lipoprotein. Metabolism 45, 1069–1079 (1996).
Sheehan, A. L. et al. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216, 321–326 (2011).
Gray, S. P. et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127, 1888–1902 (2013).
Judkins, C. P. et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am. J. Physiol. Heart Circ. Physiol. 298, H24–H32 (2010).
Douglas, G. et al. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE-/- mice. Cardiovasc. Res. 94, 20–29 (2012).
Gray, S. P. et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 36, 295–307 (2016).
Schurmann, C. et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 36, 3447–3456 (2015).
Craige, S. M. et al. Endothelial NADPH oxidase 4 protects ApoE-/- mice from atherosclerotic lesions. Free Radic. Biol. Med. 89, 1–7 (2015).
Jay, D. B. et al. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med. 45, 329–335 (2008).
Ozaki, M. et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J. Clin. Invest. 110, 331–340 (2002).
Karnewar, S. et al. Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis. Sci. Rep. 6, 24108 (2016).
San Martin, A. et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 292, H2073–H2082 (2007).
Youn, J. Y. et al. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 63, 2344–2355 (2014).
Mahmoud, A. M. et al. Nox2 contributes to hyperinsulinemia-induced redox imbalance and impaired vascular function. Redox Biol. 13, 288–300 (2017).
Wingler, K. et al. VAS2870 is a pan-NADPH oxidase inhibitor. Cell. Mol. Life Sci. 69, 3159–3160 (2012).
Kassan, M. et al. Enhanced p22phox expression impairs vascular function through p38 and ERK1/2 MAP kinase-dependent mechanisms in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 306, H972–H980 (2014).
Maxwell, S. R. & Lip, G. Y. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int. J. Cardiol. 58, 95–117 (1997).
Eltzschig, H. K. & Collard, C. D. Vascular ischaemia and reperfusion injury. Br. Med. Bull. 70, 71–86 (2004).
Brandes, R. P., Weissmann, N. & Schroder, K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 49, 687–706 (2010).
Li, Z. et al. BRG1 regulates NOX gene transcription in endothelial cells and contributes to cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3477–3486 (2018).
Sirker, A. et al. Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. J. Mol. Cell. Cardiol. 98, 11–17 (2016).
Yu, Q. et al. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J. Am. Heart Assoc. 3, e000555 (2014).
Narravula, S. & Colgan, S. P. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor α expression during hypoxia. J. Immunol. 166, 7543–7548 (2001).
Braunersreuther, V. & Jaquet, V. Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches. Curr. Pharm. Biotechnol. 13, 97–114 (2012).
Zhang, J. & Cai, H. Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS s1177/NO/DCC feed-forward mechanism. J. Mol. Cell. Cardiol. 48, 1060–1070 (2010).
Bouhidel, J. O. et al. Netrin-1 improves post-injury cardiac function in vivo via DCC/NO-dependent preservation of mitochondrial integrity, while attenuating autophagy. Biochim. Biophys. Acta 1852, 277–289 (2015).
Bouhidel, J. O., Wang, P., Li, Q. & Cai, H. Pharmacological postconditioning treatment of myocardial infarction with netrin-1. Front. Biosci. 19, 566–570 (2014).
Nguyen, A. & Cai, H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc. Natl Acad. Sci. USA 103, 6530–6535 (2006).
Li, Q., Wang, P., Ye, K. & Cai, H. Central role of SIAH inhibition in DCC-dependent cardioprotection provoked by netrin-1/NO. Proc. Natl Acad. Sci. USA 112, 899–904 (2015).
Li, Q. & Cai, H. Induction of cardioprotection by small netrin-1-derived peptides. Am. J. Physiol. Cell Physiol. 309, C100–C106 (2015).
Octavia, Y., Brunner-La Rocca, H. P. & Moens, A. L. NADPH oxidase-dependent oxidative stress in the failing heart: from pathogenic roles to therapeutic approach. Free Radic. Biol. Med. 52, 291–297 (2012).
Sirker, A., Zhang, M. & Shah, A. M. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res. Cardiol. 106, 735–747 (2011).
Maejima, Y., Kuroda, J., Matsushima, S., Ago, T. & Sadoshima, J. Regulation of myocardial growth and death by NADPH oxidase. J. Mol. Cell. Cardiol. 50, 408–416 (2011).
Zhang, M. et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl Acad. Sci. USA 107, 18121–18126 (2010).
Burgoyne, J. R., Mongue-Din, H., Eaton, P. & Shah, A. M. Redox signaling in cardiac physiology and pathology. Circ. Res. 111, 1091–1106 (2012).
Sartoretto, J. L., Kalwa, H., Pluth, M. D., Lippard, S. J. & Michel, T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc. Natl Acad. Sci. USA 108, 15792–15797 (2011).
Steinhorn, B. et al. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 9, 4044 (2018).
Bendall, J. K., Cave, A. C., Heymes, C., Gall, N. & Shah, A. M. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105, 293–296 (2002).
Li, J. M., Gall, N. P., Grieve, D. J., Chen, M. & Shah, A. M. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 40, 477–484 (2002).
Parajuli, N., Patel, V. B., Wang, W., Basu, R. & Oudit, G. Y. Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clin. Sci. 127, 331–340 (2014).
Looi, Y. H. et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51, 319–325 (2008).
Ishikawa, K. et al. Acute left ventricular unloading reduces atrial stretch and inhibits atrial arrhythmias. J. Am. Coll. Cardiol. 72, 738–750 (2018).
Takimoto, E. & Kass, D. A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49, 241–248 (2007).
Ide, T. et al. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ. Res. 86, 152–157 (2000).
Dey, S., DeMazumder, D., Sidor, A., Foster, D. B. & O’Rourke, B. Mitochondrial ROS drive sudden cardiac death and chronic proteome remodeling in heart failure. Circ. Res. 123, 356–371 (2018).
Maack, C. & Bohm, M. Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J. Am. Coll. Cardiol. 58, 83–86 (2011).
Liu, T. et al. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ. Res. 115, 44–54 (2014).
Meyer, A. J. & Dick, T. P. Fluorescent protein-based redox probes. Antioxid. Redox Signal. 13, 621–650 (2010).
Kim, Y. M. et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res. 97, 629–636 (2005).
Zhang, J. et al. NOX4-dependent hydrogen peroxide overproduction in human atrial fibrillation and HL-1 atrial cells: relationship to hypertension. Front. Physiol. 3, 140 (2012).
Schramm, A., Matusik, P., Osmenda, G. & Guzik, T. J. Targeting NADPH oxidases in vascular pharmacology. Vasc. Pharmacol. 56, 216–231 (2012).
Wingler, K. et al. NOX1, 2, 4, 5: counting out oxidative stress. Br. J. Pharmacol. 164, 866–883 (2011).
Cross, A. R. & Jones, O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237, 111–116 (1986).
Ellis, J. A., Mayer, S. J. & Jones, O. T. The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem. J. 251, 887–891 (1988).
Simons, J. M., Hart, B. A., Ip Vai Ching, T. R., Van Dijk, H. & Labadie, R. P. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic. Biol. Med. 8, 251–258 (1990).
Suzuki, Y., Wang, W., Vu, T. H. & Raffin, T. A. Effect of NADPH oxidase inhibition on endothelial cell ELAM-1 mRNA expression. Biochem. Biophys. Res. Commun. 184, 1339–1343 (1992).
Garrido-Urbani, S. et al. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARα mediated mechanism. PLOS ONE 6, e14665 (2011).
Sedeek, M. et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 299, F1348–F1358 (2010).
Aoyama, T. et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56, 2316–2327 (2012).
O’Donnell, V. B., Smith, G. C. & Jones, O. T. Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Mol. Pharmacol. 46, 778–785 (1994).
O’Donnell, B. V., Tew, D. G., Jones, O. T. & England, P. J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 290, 41–49 (1993).
Gianni, D. et al. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem. Biol. 5, 981–993 (2010).
Altenhofer, S. et al. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell. Mol. Life Sci. 69, 2327–2343 (2012).
Maraldi, T. Natural compounds as modulators of NADPH oxidases. Oxid. Med. Cell. Longev. 2013, 271602 (2013).
Barbieri, S. S. et al. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic. Biol. Med. 37, 156–165 (2004).
Stolk, J., Hiltermann, T. J., Dijkman, J. H. & Verhoeven, A. J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 11, 95–102 (1994).
Williams, H. C. & Griendling, K. K. NADPH oxidase inhibitors: new antihypertensive agents? J. Cardiovasc. Pharmacol. 50, 9–16 (2007).
Tanriverdi, L. H. et al. Inhibition of NADPH oxidase by apocynin promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress in rats. Free. Radic. Res. 51, 772–786 (2017).
Drummond, G. R., Selemidis, S., Griendling, K. K. & Sobey, C. G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 10, 453–471 (2011).
Remold-O’Donnell, E. & Parent, D. Downregulation of neutrophil CD43 by opsonized zymosan. Blood 85, 337–342 (1995).
Diatchuk, V., Lotan, O., Koshkin, V., Wikstroem, P. & Pick, E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J. Biol. Chem. 272, 13292–13301 (1997).
Wartenberg, M. et al. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett. 579, 4541–4549 (2005).
Cayatte, A. J. et al. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets NADPH oxidase. Arterioscler. Thromb. Vasc. Biol. 21, 1577–1584 (2001).
Zang, M. et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55, 2180–2191 (2006).
Delbosc, S. et al. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J. Cardiovasc. Pharmacol. 40, 611–617 (2002).
Wassmann, S. et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 22, 300–305 (2002).
Wei, Y. M. et al. Attenuation by statins of membrane raft-redox signaling in coronary arterial endothelium. J. Pharmacol. Exp. Ther. 345, 170–179 (2013).
Kwok, J. M. F., Ma, C. C. H. & Ma, S. Recent development in the effects of statins on cardiovascular disease through Rac1 and NADPH oxidase. Vasc. Pharmacol. 58, 21–30 (2013).
Shiga, N. et al. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ. Res. 96, 1014–1021 (2005).
Gao, Y., Dickerson, J. B., Guo, F., Zheng, J. & Zheng, Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl Acad. Sci. USA 101, 7618–7623 (2004).
Youn, J. Y., Nguyen, A. & Cai, H. Inhibition of XO or NOX attenuates diethylstilbestrol-induced endothelial nitric oxide deficiency without affecting its effects on LNCaP cell invasion and apoptosis. Clin. Sci. 123, 509–518 (2012).
ten Freyhaus, H. et al. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 71, 331–341 (2006).
Stielow, C. et al. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem. Biophys. Res. Commun. 344, 200–205 (2006).
Wind, S. et al. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br. J. Pharmacol. 161, 885–898 (2010).
Seredenina, T. et al. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic. Biol. Med. 86, 239–249 (2015).
Perry, B. N. et al. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J. Invest. Dermatol. 126, 2316–2322 (2006).
Munson, J. M. et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl Med. 4, 127ra36 (2012).
Bhandarkar, S. S. et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J. Clin. Invest. 119, 2359–2365 (2009).
Rey, F. E., Cifuentes, M. E., Kiarash, A., Quinn, M. T. & Pagano, P. J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2 – and systolic blood pressure in mice. Circ. Res. 89, 408–414 (2001).
Csanyi, G. et al. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic. Biol. Med. 51, 1116–1125 (2011).
Ranayhossaini, D. J. et al. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J. Biol. Chem. 288, 36437–36450 (2013).
Cifuentes-Pagano, E., Csanyi, G. & Pagano, P. J. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell. Mol. Life Sci. 69, 2315–2325 (2012).
Laleu, B. et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J. Med. Chem. 53, 7715–7730 (2010).
Anvari, E., Wikstrom, P., Walum, E. & Welsh, N. The novel NADPH oxidase 4 inhibitor GLX351322 counteracts glucose intolerance in high-fat diet-treated C57BL/6 mice. Free Radic. Res. 49, 1308–1318 (2015).
Wang, X. et al. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLOS ONE 13, e0204271 (2018).
Hirano, K. et al. Discovery of gsk2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid. Redox Signal. 23, 358–374 (2015).
Musset, B. et al. NOX5 in human spermatozoa: expression, function, and regulation. J. Biol. Chem. 287, 9376–9388 (2012).
Jiang, J. X. et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic. Biol. Med. 53, 289–296 (2012).
Schildknecht, S. et al. The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr. Med. Chem. 21, 365–376 (2014).
Strengert, M. et al. Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza A virus infection. Antioxid. Redox Signal. 20, 2695–2709 (2014).
Gorin, Y. et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 308, F1276–F1287 (2015).
Teixeira, G. et al. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br. J. Pharmacol. 174, 1647–1669 (2017).
Vendrov, A. E. et al. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J. Biol. Chem. 285, 26545–26557 (2010).
Di Marco, E. et al. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe -/- mice. Diabetologia 57, 633–642 (2014).
Joo, J. H. et al. A novel pyrazole derivative protects from ovariectomy-induced osteoporosis through the inhibition of NADPH oxidase. Sci. Rep. 6, 22389 (2016).
Cha, J. J. et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab. Invest. 97, 419–431 (2017).
Dorotea, D. et al. A pan-NADPH oxidase inhibitor ameliorates kidney injury in type 1 diabetic rats. Pharmacology 102, 180–189 (2018).
Luxen, S., Belinsky, S. A. & Knaus, U. G. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 68, 1037–1045 (2008).
Shames, D. S. et al. A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLOS MED 3, e486 (2006).
Hayes, P. & Knaus, U. G. Balancing reactive oxygen species in the epigenome: NADPH oxidases as target and perpetrator. Antioxid. Redox Signal. 18, 1937–1945 (2013).
Kikuchi, H., Kuribayashi, F., Kiwaki, N., Takami, Y. & Nakayama, T. GCN5 regulates the superoxide-generating system in leukocytes via controlling gp91-phox gene expression. J. Immunol. 186, 3015–3022 (2011).
Siuda, D. et al. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res. Cardiol. 107, 283 (2012).
Zelko, I. N. & Folz, R. J. Regulation of oxidative stress in pulmonary artery endothelium: modulation of extracellular superoxide dismutase and NOX4 expression using histone deacetylase class I inhibitors. Am. J. Respir. Cell Mol. Biol. 53, 513–524 (2015).
Chen, F. et al. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radic. Biol. Med. 99, 167–178 (2016).
Manea, S. A. et al. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol. 16, 332–343 (2018).
Duraisamy, A. J., Mishra, M., Kowluru, A. & Kowluru, R. A. Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 59, 4831–4840 (2018).
Yu, L. et al. Megakaryocytic leukemia 1 bridges epigenetic activation of NADPH oxidase in macrophages to cardiac ischemia-reperfusion injury. Circulation 138, 2820–2836 (2018).
Murdoch, C. E. et al. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J. Am. Coll. Cardiol. 63, 2734–2741 (2014).
Pollock, J. D. et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9, 202–209 (1995).
Jackson, S. H., Gallin, J. I. & Holland, S. M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182, 751–758 (1995).
Acknowledgements
The authors are supported by an AHA Postdoctoral Fellowship Award #14POST20380996 (Y.Z.), NIH National Heart, Lung, and Blood Institute (NHLBI) grants HL077440, HL088975 and HL119968, and an AHA Established Investigator Award 12EIA8990025 (H.C.).
Author information
Authors and Affiliations
Contributions
All the authors researched data for the article and wrote the manuscript. Y.Z. and H.C. discussed the content of the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
RCSB Protein Data Bank: https://www.rcsb.org/
Rights and permissions
About this article
Cite this article
Zhang, Y., Murugesan, P., Huang, K. et al. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol 17, 170–194 (2020). https://doi.org/10.1038/s41569-019-0260-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-019-0260-8
This article is cited by
-
Endothelial dysfunction due to eNOS uncoupling: molecular mechanisms as potential therapeutic targets
Cellular & Molecular Biology Letters (2023)
-
Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways
Journal of Translational Medicine (2023)
-
Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Fibroblast growth factor 18 alleviates stress-induced pathological cardiac hypertrophy in male mice
Nature Communications (2023)
-
The role of HDAC3 and its inhibitors in regulation of oxidative stress and chronic diseases
Cell Death Discovery (2023)