Key Points
-
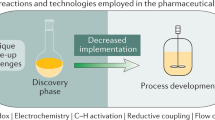
Lead compound optimization and medicinal chemistry are known to be the bottlenecks in the drug discovery process, and so a need arises for technologies that allow more rapid synthesis of chemical substances. One such high-speed technology is microwave-assisted organic synthesis (MAOS).
-
Microwave heating, compared with conventional heating (which means heating with an external source such as an oil-bath) is much more efficient because the reaction mixture is heated internally by direct coupling of microwave energy with polar molecules (solvents, reagents and catalysts, for example). This allows faster heating to higher temperatures using sealed-vessel technology.
-
This enabling technology has gained significant influence in the lead optimization and lead generation processes in the pharmaceutical industry because microwave heating dramatically reduces reaction times from days or hours to minutes or seconds.
-
Many reaction parameters, such as time, temperature, solvents, concentration or catalysts, can now be evaluated in a fraction of the time, compared with conventional heating, in the optimization step.
-
Compound libraries can be rapidly synthesized either in a parallel or sequential automated format for lead discovery or structure–activity studies.
-
MAOS can also be combined with solid- or fluorous-phase synthesis or solid-supported solution-phase synthesis, respectively, to achieve simpler product isolation and purification.
-
Several examples for high-throughput microwave synthesis using parallel or sequential library production as well as lead optimization and generation studies are covered in this review.
Abstract
In the past few years, using microwave energy to heat and drive chemical reactions has become increasingly popular in the medicinal chemistry community. First described 20 years ago, this non-classical heating method has matured from a laboratory curiosity to an established technique that is heavily used in academia and industry. One of the many advantages of using rapid 'microwave flash heating' for chemical synthesis is the dramatic reduction in reaction times — from days and hours to minutes and seconds. As will be discussed here, there are good reasons why many pharmaceutical companies are incorporating microwave chemistry into their drug discovery efforts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kappe, C. O. & Stadler, A. Microwaves in Organic and Medicinal Chemistry (Wiley-VCH, Weinheim, 2005). This book provides the most comprehensive coverage of microwave-assisted organic synthesis, as well as a complete list of all other reviews.
Tierney, J. P. & Lidström, P. (eds) Microwave Assisted Organic Synthesis (Blackwell, Oxford, 2005).
Loupy, A. (ed.) Microwaves in Organic Synthesis (Wiley-VCH, Weinheim, 2002).
Hayes, B. L. Microwave Synthesis: Chemistry at the Speed of Light (CEM Publishing, Matthews, 2002).
Kappe, C. O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 43, 6250–6284 (2004).
Hayes, B. L. Recent advances in microwave-assisted synthesis. Aldrichim. Acta 37, 66–77 (2004).
Leadbeater, N. E. Fast, easy, clean chemistry by using water as a solvent and microwave heating: the Suzuki coupling as an illustration. Chem. Commun. 2881–2902 (2005).
Baghurst, D. R. & Mingos, D. M. P. Application of microwave dielectric heating effects to synthetic problems in chemistry. Chem. Soc. Rev. 20, 1–47 (1991).
Gabriel, C., Gabriel, S., Grant, E. H., Halstead, B. S. & Mingos, D. M. P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 27, 213–223 (1998). References 8 and 9 provide detailed reviews explaining the quite complex phenomena associated with microwave dielectric heating to synthetic chemists.
Gronnow, M. J., White, R. J., Clark, J. H. & Macquarrie, D. J. Energy efficiency in chemical reactions: a comparative study of different reaction techniques. Org. Process Res. Dev. 9, 516–518 (2005).
Perreux, L. & Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 57, 9199–9223 (2001).
De La Hoz, A., Diaz-Ortiz, A. & Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 34, 164–178 (2005). A review summarizing and evaluating the latest theories on the existence of non-thermal and specific microwave effects.
Gedye, R. et al. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 27, 279–282 (1986). This seminal publication describes for the first time the use of domestic microwave ovens for synthetic organic transformations.
Giguere, R. J., Bray, T. L., Duncan, S. M. & Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 27, 4945–4958 (1986).
Glasnov, T. N., Stadlbauer, W. & Kappe, C. O. Microwave-assisted multistep synthesis of functionalized 4-arylquinolin-2(1H)-ones using palladium-catalyzed cross-coupling chemistry. J. Org. Chem. 70, 3864–3870 (2005).
Porcheddu, A., Ruda, G. F., Sega, A. & Taddei, M. A new, rapid, general procedure for the synthesis of organic molecules supported on methoxy-polyethylene glycol (MeOPEG) under microwave irradiation conditions. Eur. J. Org. Chem. 907–912 (2003).
Alexandre, F. -R., Berecibar, A., Wrigglesworth, R. & Besson, T. Novel series of 8H-quinazolino[4,3-b]quinazolin-8-ones via two Niementowski condensations. Tetrahedron 59, 1413–1419 (2003).
Pemberton, N., Åberg, V., Almstedt, H., Westermark, A. & Almqvist, F. Microwave-assisted synthesis of highly substituted aminomethylated 2-pyridones. J. Org. Chem. 69, 7830–7835 (2004).
Stadler, A. & Kappe, C. O. Automated library generation using sequential microwave-assisted chemistry. Application toward the Biginelli multicomponent condensation. J. Comb. Chem. 3, 624–630 (2001). This publication introduces and describes in full detail the concept of automated library generation using a robotized single-mode microwave synthesizer.
Larhed, M. & Hallberg, A. Microwave-assisted high-speed chemistry: a new technique in drug discovery. Drug Discov. Today 6, 406–416 (2001). An excellent review aimed at the medicinal chemist, summarizing many of the concepts and ideas that have subsequently evolved in this area.
Kappe, C. O. & Stadler, A. Building dihydropyrimidine libraries via microwave-assisted Biginelli multicomponent reactions. Method. Enzym. 369, 197–223 (2003).
Kappe, C. O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 33, 879–888 (2000).
Kappe, C. O. Biologically active dihydropyrimidones of the Biginelli-type. A literature survey. Eur. J. Med. Chem. 35, 1043–1052 (2000).
Tye, H. Application of statistical 'design of experiments' methods in drug discovery. Drug Discov. Today 9, 485–491 (2004). Describes how microwave synthesis and experimental design can be efficiently interfaced with drug discovery and reaction optimization.
Evans, M. D. et al. The accelerated development of an optimized synthesis of 1,2, 4-oxadiazoles: application of microwave irradiation and statistical design of experiments. Tetrahedron Lett. 44, 9337–9341 (2003).
Tye, H. & Whittaker, M. Use of a design of experiments approach for the optimization of a microwave assisted Ugi reaction. Org. Biomol. Chem. 2, 813–815 (2004).
McLean, N. J., Tye, H. & Whittaker, M. Microwave assisted Petasis boronic-Mannich reactions. Tetrahedron Lett. 45, 993–995 (2004).
Sarko, C. R. in Microwave Assisted Organic Synthesis (eds Tierney, J. P. & Lidström, P.) 222–236 (Blackwell, Oxford, 2005).
Cotterill, I. C. et al. Microwave-assisted combinatorial chemistry. Synthesis of substituted pyridines. Tetrahedron Lett. 39, 1117–1120 (1998). This paper describes for the first time the utilization of microtitre plates for high-speed parallel synthesis under microwave conditions.
Nüchter, M. & Ondruschka, B. Tools for microwave-assisted parallel synthesis and combinatorial chemistry. Mol. Diversity 7, 253–264 (2003).
Coleman, C. M., MacElroy, J. M. D., Gallagher, J. F. & O'Shea, D. F. Microwave parallel library generation: comparison of a conventional- and microwave-generated substituted 4(5)-sulfanyl-1H-imidazole library. J. Comb. Chem. 4, 87–93 (2002).
Glass, B. M. & Combs, A. P. in High-Throughput Synthesis. Principles and Practices (ed. Sucholeiki, I.) 123–128 (Marcel Dekker, New York, 2001).
Alcázar, J. Reproducibility across microwave instruments: preparation of a set of 24 compounds on a multiwell plate under temperature-controlled conditions. J. Comb. Chem. 7, 353–355 (2005).
Tan, D. S. Diversity-oriented synthesis: exploring the intersections between chemistry and biology. Nature Chem. Biol. 1, 74–84 (2005).
Zhao, Z., Leister, W. H., Strauss, K. A., Wisnoski, D. D. & Lindsley, C. W. Broadening the scope of 1,2,4-triazine synthesis by the application of microwave technology. Tetrahedron Lett. 44, 1123–1127 (2003).
Wolkenberg, S. E. et al. Efficient synthesis of imidazoles from aldehydes and 1,2-diketones using microwave irradiation. Org. Lett. 6, 1453–1456 (2004).
Zhao, Z. et al. General microwave-assisted protocols for the expedient synthesis of quinoxalines and heterocyclic pyrazines. Tetrahedron Lett. 45, 4873–4876 (2004).
Lindsley, C. W. et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 15, 761–764 (2005).
Lindsley, C. W., Wisnoski, D. D., Wang, Y., Leister, W. H. & Zhao, Z. A one pot microwave-mediated synthesis of the basic canthine skeleton: expedient access to unnatural β-carboline alkaloids. Tetrahedron Lett. 44, 4495–4498 (2003).
Zhao, Z. et al. Discovery of 2, 3, 5-trisubstituted pyridine derivatives as potent Akt1 and Akt2 dual inhibitors. Bioorg. Med. Chem. Lett. 15, 905–909 (2005).
Nöteberg, D., Schaal, W., Hamelink, E., Vrang, L. & Larhed, M. High-speed optimization of inhibitors of the malarial proteases plasmepsin I and II. J. Comb. Chem. 5, 456–464 (2003).
Gardner, M. J. et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 (2002).
Banerjee, R. et al. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl Acad. Sci. USA 99, 990–995 (2002).
Berry, C. Plasmepsins as antimalarial targets. Curr. Opin. Drug Discovery Dev. 3, 624–629 (2000).
Boss, C. et al. Inhibitors of the Plasmodium falciparum parasite aspartic protease plasmepsin II as potential malarial agents. Curr. Med. Chem. 10, 883–907 (2003).
Nöteberg, D. et al. Design and synthesis of plasmepsin I and plasmepsin II inhibitors with activity in Plasmodium falciparum-infected cultured human erythrocytes. J. Med. Chem. 46, 734–746 (2003).
Ersmark, K., Larhed, M. & Wannberg, J. Microwave-enhanced medicinal chemistry: a high-speed opportunity for convenient preparation of protease inhibitors. Curr. Opin. Drug. Discov. Develop. 7, 417–427 (2004). A stimulating account on the use of microwave synthesis in medicinal chemistry from an academic perspective, written by a pioneer in this area. See also reference 20.
Ersmark, E. et al. Potent inhibitors of the Plasmodium falciparum enzyme plasmepsin I and II devoid of cathepsin D inhibitory activity. J. Med. Chem. 47, 110–122 (2004).
Wannberg, J. et al. High-speed synthesis of potent C2-symmetric HIV-1 protease inhibitors by in-situ aminocarbonylations. J. Comb. Chem. 7, 611–617 (2005).
Ax, A. et al. Cyclic sulfamide HIV-1 protease inhibitors, with sidechains spanning from P2/P2' to P1/P1'. Bioorg. Med. Chem. 13, 755–764 (2005).
Wan, Y. et al. Design, synthesis and biological evaluation of the first selective nonpeptide AT2 receptor antagonist. J. Med. Chem. 47, 5995–6008 (2004).
Organ, M. S., Mayer, S., Lepifre, F., N'Zemba, B. & Khatri, J. Combining the use of solid-supported transition metal catalysis with microwave irradiation in solution-phase parallel library synthesis. Mol. Diversity 7, 211–227 (2003).
Coats, S. J. et al. Parallel methods for the preparation and SAR exploration of N-ethyl-4-[(8-alkyl-8-azabicyclo[3.2.1]-oct-3-ylidene)-aryl-methyl]-benzamides, powerful μ and δ opioid agonists. Bioorg. Med. Chem. Lett. 14, 5493–5498 (2004).
Vasudevan, A., Villamil, C. I. & Djuric, S. W. Synthesis of diazepinones via intramolecular transamidation. Org. Lett. 6, 3361–3364 (2004).
Kempson, J. et al. Fused pyrimidine based inhibitors of phosphodiesterase 7 (PDE7): synthesis and initial structure–activity relationships. Bioorg. Med. Chem. Lett. 15, 1829–1833 (2005).
Lehmann, F., Currier, E. A., Olsson, R., Hacksell, U. & Luthman, K. Isochromanone-based urotensin-II receptor agonists. Bioorg. Med. Chem. 13, 3057–3068 (2005).
Santagada, V. et al. Efficient microwave combinatorial parallel and nonparallel synthesis of N-alkylated glycine methyl esters as peptide building blocks. J. Comb. Chem. 7, 618–621 (2005).
Dai, W. -M., Wang, X. & Ma, C. Microwave-assisted one-pot regioselective synthesis of 2-alkyl-3,4-dihydro-3-oxo-2H-1, 4-benzoxazines. Tetrahedron 61, 6879–6885 (2005).
Wang, Y., Miller, R. L., Sauer, D. R. & Djuric, S. W. Rapid and efficient synthesis of 1,2,4-oxadiazoles utilizing polymer-supported reagents under microwave heating. Org. Lett. 7, 925–928 (2005).
Holmberg, P., Tedenborg, L., Rosqvist, S. & Johansson, A. M. Novel 3-aminochromans as potential pharmacological tools for the serotonin 5-HT7 receptor. Bioorg. Med. Chem. Lett. 15, 747–750 (2005).
Seijas, J. A., Vàzquez-Tato, M. P. & Carballido-Reboredo, R. Solvent-free synthesis of functionalized flavones under microwave irradiation. J. Org. Chem. 70, 2855–2858 (2005).
Liu, J. -F. et al. Novel one-pot total syntheses of deoxyvasicinone, mackinazolinone, isaindigotone, and their derivatives promoted by microwave irradiation. Org. Lett. 7, 3363–3366 (2005).
Appukkuttan, P., Dehaen, W. & Van der Eycken, E. Microwave-enhanced synthesis of N-shifted buflavine analogues via a Suzuki-ring-closing metathesis protocol. Org. Lett. 7, 2723–2726 (2005).
Vvedensky, V. Y. et al. Microwave-mediated reactions of 3-aminomethylpyridines with acetylenedicarboxylates. A novel synthetic route to dihydronaphthyridines and naphthyridine-1-ones. Tetrahedron Lett. 46, 3953–3956 (2005).
Cheung, W. S., Patch, R. J. & Player, M. R. A tandem Heck-carbocyclization/Suzuki-coupling approach to the stereoselective syntheses of asymmetric 3,3-(diarylmethylene)indolinones. J. Org. Chem. 70, 3741–3744 (2005).
Alterman, M. et al. Design and fast synthesis of C-terminal duplicated potent C2-symmetric P1/P1′-modified HIV-1 protease inhibitors. J. Med. Chem. 42, 3835–3844 (1999). This is probably the first publication on the use of controlled microwave heating in a lead optimization project.
Zaragoza Dörwald, F. Organic Synthesis on Solid Phase (Wiley-VCH, Weinheim, 2002).
Gladysz, J. A., Curran, D. P. & Horváth, I. (eds). Handbook of Fluorous Chemistry (Wiley-VCH, Weinheim, 2005).
Ley, S. V. & Baxendale, I. R. New tools and concepts for modern organic synthesis. Nature Rev. Drug Discov. 1, 573–586 (2002).
Frutos Hoener, A. P., Henkel, B. & Gauvin, J. -C. Novel one-pot microwave assisted Gewald synthesis of 2-acyl amino thiophenes on solid support. Synlett 63–66 (2003).
Vallin, K. S. A., Zhang, Q., Larhed, M., Curran, D. P. & Hallberg, A. A new regioselective Heck vinylation with enamides. Synthesis and investigation of fluorous-tagged bidentate ligands for fast separation. J. Org. Chem. 68, 6639–6645 (2003).
Sauer, D. R., Kalvin, D. & Phelan, K. M. Microwave-assisted synthesis utilizing supported reagents: A rapid and efficient acylation procedure. Org. Lett. 5, 4721–4724 (2003).
Erdelyi, M. & Gogoll, A. Rapid microwave-assisted solid phase peptide synthesis. Synthesis 1592–1596 (2002).
Matsushita, T., Hinou, H., Kurogochi, M., Shimizu, H. & Nishimura, S. -I. Rapid microwave-assisted solid-phase glycopeptide synthesis. Org. Lett. 7, 877–880 (2005).
Murray, J. K. & Gellman, S. H. Application of microwave irradiation to the synthesis of 14-helical β-peptides. Org. Lett. 7, 1517–1520 (2005).
Murray, J. K. et al. Efficient synthesis of a β-peptide combinatorial library with microwave irradiation, J. Am. Chem. Soc. 127, 13271–13280 (2005). An excellent article describing in full detail the concept of microwave-assisted solid-phase peptide synthesis under controlled conditions.
Scharn, D., Wenschuh, H., Reineke, U., Schneider-Mergener, J. & Germeroth, L. Spatially addressed synthesis of amino- and amino-oxy-substituted 1,3,5-triazine arrays on polymeric membranes. J. Comb. Chem. 2, 361–369 (2000).
Scharn, D., Germeroth, L., Schneider-Mergener, J. & Wenschuh, H. Sequential nucleophilic substitution on halogenated triazines, pyrimidines, and purines: a novel approach to cyclic peptidomimetics. J. Org. Chem. 66, 507–513 (2001).
Bowman, M. D., Jeske, R. C. & Blackwell, H. E. Microwave-accelerated SPOT-synthesis on cellulose supports. Org. Lett. 6, 2019–2022 (2004).
Watts, P. Continuous flow microreactors for drug discovery. Curr. Opin. Drug Discov. Develop. 7, 807–812 (2004)
Watts, P. & Haswell, S. W. Microfluidic combinatorial chemistry. Curr. Opin. Chem. Biol. 7, 380–387 (2003).
He, P., Haswell, S. J. & Fletcher, P. D. I. Microwave heating of heterogeneously catalyzed Suzuki reactions in a micro reactor. Lab Chip 4, 38–41 (2004).
He, P., Haswell, S. J. & Fletcher, P. D. I. Microwave-assisted Suzuki reactions in a continuous flow capillary reactor. Appl. Catal. A. 274, 111–114 (2004).
He, P., Haswell, S. J. & Fletcher, P. D. I. Efficiency, monitoring and control of microwave heating within a continuous flow capillary reactor. Sensors Actuators B 105, 516–520 (2005).
Comer, E. & Organ, M. G. A microreactor for microwave-assisted capillary (continuous flow) organic synthesis. J. Am. Chem. Soc. 127, 8160–8167 (2005).
Comer, E. & Organ, M. G. A. Microcapillary system for simultaneous, parallel microwave assisted synthesis. Chem. Eur. J. 11, in press (2005).
Pivonka, D. E. & Empfield, J. R. Real-time in situ Raman analysis of microwave-assisted organic reactions. Appl. Spect. 58, 41–46 (2004).
Stadler, A. et al. Scalability of microwave-assisted organic synthesis. From single-mode to multimode parallel batch reactors. Org. Process Res. Dev. 7, 707–716 (2003).
Deetlefs, M. & Seddon, K. R. Improved preparation of ionic liquids using microwave irradiation. Green Chem. 5, 181–186 (2003).
Lehmann, F., Pilotti, Å. & Luthman, K. Efficient large scale microwave assisted Mannich reactions using substituted acetophenones. Mol. Diversity 7, 145–152 (2003).
Shackelford, S. A. et al. Electrophilic tetraalkylammonium nitrate nitration. II. Improved anhydrous aromatic and heteroaromatic mononitration with tetramethylammonium nitrate and triflic anhydride, including selected microwave examples. J. Org. Chem. 68, 267–275 (2003). References 88 and 91 summarize some of the concepts and problems of scaling-up microwave-assisted reactions.
Roberts, B. A. & Strauss C. R. in Microwave Assisted Organic Synthesis (eds Tierney, J. P. & Lidström, P.) 237–271 (Blackwell, Oxford, 2005).
Shieh, W. -C., Dell, S. & Repiĉ, O. Large scale microwave-accelerated esterification of carboxylic acids with dimethyl carbonate. Tetrahedron Lett. 43, 5607–5609 (2002).
Shieh, W. -C., Dell, S. & Repiĉ, O. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and microwave-accelerated green chemistry in methylation of phenols, indoles, and benzimidazoles with dimethyl carbonate. Org. Lett. 3, 4279–4281 (2001).
Wilson, N. S., Sarko, C. R. & Roth, G. Development and applications of a practical continuous flow microwave cell. Org. Process Res. Dev. 8, 535–538 (2004).
Arvela, R. K., Leadbeater, N. E. & Collins, M. J. Jr. Automated batch scale-up of microwave-promoted Suzuki and Heck coupling reactions in water using ultra-low metal catalyst concentrations, Tetrahedron 61, 9349–9355 (2005).
Loones, K. T. J., Maes, B. U. W., Rombouts, G., Hostyn, S. & Diels, G. Microwave-assisted organic synthesis: scale-up of palladium-catalyzed aminations using single-mode and multi-mode microwave equipment, Tetrahedron 61, 10338–10348 (2005).
Howarth, P. & Lockwood, M. Reactor Design 1: Come of Age. The Chemical Engineer 756, 29–31 (2004).
Acknowledgements
The work of the Kappe research laboratories in the area of microwave chemistry has been supported by the Austrian Science Fund, the 'Jubiläumsfonds der Österreichischen Nationalbank', the European Union COST program, the Austrian Academic Exchange Service, the University of Graz and various industrial contributors. We wish to thank all members of the Microwave Synthesis (MAOS) Lab in Graz for their essential contributions to microwave chemistry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- Combinatorial chemistry
-
The generation of large collections, or 'libraries', of compounds by synthesizing all possible combinations of a set of smaller chemical structures.
- Compound libraries
-
Large collections of compounds (hundreds to millions), often synthesized through reactions performed simultaneously (in parallel) or by using combinatorial chemistry principles.
- Chemical space
-
The space spanned by all possible (energetically stable) combinations of atoms and topologies in molecules.
- Microwave irradiation
-
Electromagnetic irradiation in the frequency range of 0.3–300 GHz, corresponding to wavelengths of 1 cm–1 m. All microwave reactors for chemical synthesis operate at a frequency of 2.45 GHz (corresponding to a wavelength of 12.25 cm) to avoid interference with telecommunication and cellular phone frequencies.
- Arrhenius law
-
The relationship between reaction rate and temperature. The rate of a chemical reaction increases when the temperature is raised according to: k = A exp(−Ea/RT).
- Microtitre plates
-
Sample holders used for synthesis, storage, analysis and screening of compound libraries. The plates typically have 6, 24, 96, 384 or even 1,536 sample wells arranged in a 2:3 rectangular matrix.
- Design of experiment
-
(DoE). The use of factorial experiments instead of the one-factor-at-a-time method for the optimization of reaction conditions. This allows studying the effect of each factor on the response variable, while requiring fewer observations than by conducting separate experiments for each factor independently.
- Dielectric properties
-
The ability of a specific sub-stance to convert electro-magnetic energy into heat at a given frequency and temp-erature is determined by the 'loss tangent', tan δ. A reaction medium with a high (>0.5) tan δ value is required for efficient absorption and rapid heating.
- Diversity-oriented synthesis
-
(DOS). Efficient synthesis of a collection (combinatorial library) of structurally diverse and complex small molecules that differ in stereochemistry, functional groups and molecular framework. Rather than being directed toward a single biological target, DOS libraries can be used to identify new ligands for a variety of targets.
- Microreactor
-
Continuous-flow device that consists of a series of interconnecting channels (50–500 μm diameter) and facilitates the performance of chemical reactions on a micro-litre scale. Independent reactants are brought together through different feeder channels to the main channel, where they mix and react as they travel to a reservoir where the products are collected.
Rights and permissions
About this article
Cite this article
Kappe, C., Dallinger, D. The impact of microwave synthesis on drug discovery. Nat Rev Drug Discov 5, 51–63 (2006). https://doi.org/10.1038/nrd1926
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd1926
This article is cited by
-
Microwave-assisted synthesis of double-headed derivatives of (4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)-ethan-1-ol and study of their biological activity
Research on Chemical Intermediates (2021)
-
Nano-Co3O4-catalyzed microwave-assisted one-pot synthesis of some seleno [2 , 3-b ] pyridine/quinoline derivatives
Research on Chemical Intermediates (2021)
-
Microwave-accelerated diastereoselective catalyst-free one-pot four-component synthesis of 2-(N-carbamoylacetamide)-substituted 2,3-dihydrothiophenes in glycerol
Molecular Diversity (2020)
-
Design and synthesis of anticancer 1-hydroxynaphthalene-2-carboxanilides with a p53 independent mechanism of action
Scientific Reports (2019)
-
Microwave irradiation directly excites semiconductor catalyst to produce electric current or electron-holes pairs
Scientific Reports (2019)