Key Points
-
Although immunosuppressive therapy is effective at present, toxicity remains an important problem.
-
Many of the existing immunosuppressive agents are directed against ubiquitous targets and therefore have side effects that are unrelated to immunosuppression. Consequently, generating drugs against molecules with restricted expression and/or function might be advantageous.
-
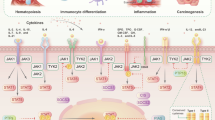
The Janus kinase JAK3 is crucial for signalling by key immunoregulatory cytokines, but has restricted expression and function. This is best illustrated by patients with mutations of the gene encoding this kinase: such children have severe combined immunodeficiency but do not have abnormalities outside of the immune system. This phenotype suggests that JAK3 might be an ideal target.
-
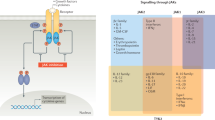
A selective JAK3 inhibitor, CP-690,550, has now been generated to effectively block immune responses both in vitro and in vivo. Other JAK3 inhibitors have been previously described, but none are as potent or selective as CP-690,550. This drug is effective in models of transplant rejection and is not associated with the toxicities that are seen with other immunosuppressive agents. A JAK3 inhibitor is likely to have uses in many settings beyond transplantation, including autoimmune disease and possibly haematopoietic malignancy.
-
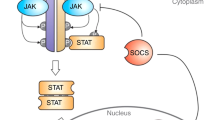
Targeting other JAKS and other elements in the JAK/STAT pathway is also conceptually appealing. On the basis of the phenotype that is associated with TYK2 deficiency, a TYK2 antagonist might be useful in inhibiting diseases that are characterized by the activation of TH1 cells. Given their importance in malignant transformation and immunoregulation, STAT proteins have received considerable attention as therapeutic targets and STAT inhibitors are being studied at present. SOCs proteins are cytokine-induced feedback inhibitors of signalling, which can also be considered as potential targets.
Abstract
Thousands of organs are transplanted each year and millions of people suffer from autoimmune diseases, which creates a need for an armamentarium of immunosuppressive drugs. Unfortunately, immunosuppressants have unwanted side effects owing, in part, to the fact that they have ubiquitous molecular targets. Cytokines have emerged as important controllers of the immune response, and work during the past decade has identified Janus kinases (JAKs) and signal transducers, and activators of transcription (STATs), as crucial intracellular elements in cytokine signalling. Here, we discuss the potential of the JAK/STAT pathway as a target for new immunosuppressants. In particular, the inhibition of JAK3 seems to be an excellent strategy, because of the selective expression and precise functions of this kinase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hong, J. C. & Kahan, B. D. Immunosuppressive agents in organ transplantation: past, present, and future. Semin. Nephrol. 20, 108–125 (2000).
Boulay, J. L., O'Shea, J. J. & Paul, W. E. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity 19, 159–163 (2003).
Glimcher, L. H. Lineage commitment in lymphocytes: controlling the immune response. J. Clin. Invest. 108, 25–30 (2001).
Murphy, K. M. & Reiner, S. L. The lineage decisions of helper T cells. Nature Rev. Immunol. 2, 933–944 (2002). An excellent review of the control of helper T-cell differentiation by cytokines and transcription factors.
Agnello, D. et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 23, 147–161 (2003).
Darnell, J. E. Jr., Kerr, I. M. & Stark, G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994). An outstanding review of the discovery of the JAK/STAT pathway by pioneers in the field.
Ihle, J. N. The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv. Immunol. 60, 1–35 (1995).
O'Shea, J. J. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity 7, 1–11 (1997).
Leonard, W. J. & O'Shea, J. J. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16, 293–322 (1998).
O'Shea, J. J., Gadina, M. & Schreiber, R. D. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109 (Suppl.), S121–S131 (2002).
Chen, M. et al. Complex effects of naturally occurring mutations in the JAK3 pseudokinase domain: evidence for interactions between the kinase and pseudokinase domains. Mol. Cell Biol. 20, 947–956 (2000).
Saharinen, P., Takaluoma, K. & Silvennoinen, O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell Biol. 20, 3387–3395 (2000).
Zhou, Y. J. et al. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8, 959–969 (2001).
Ragimbeau, J. et al. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 22, 537–547 (2003).
Velazquez, L., Fellous, M., Stark, G. R. & Pellegrini, S. A protein tyrosine kinase in the interferon α/β signaling pathway. Cell 70, 313–322 (1992). A seminal study in the JAK/STAT field that gave the first clues as to the function of JAKs.
Witthuhn, B. A. et al. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature 370, 153–157 (1994).
Kawamura, M. et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc. Natl Acad. Sci. USA 91, 6374–6378 (1994).
Johnston, J. A. et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 370, 151–153 (1994). The first report on the cloning and function of JAK3.
Noguchi, M. et al. Interleukin-2 receptor γ-chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73, 147–157 (1993). A pivotal study that showed that X-SCID is the result of mutations in what became known as the common γ-chain.
Asao, H. et al. Cutting edge: the common γ-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167, 1–5 (2001).
Leonard, W. J., Noguchi, M., Russell, S. M. & McBride, O. W. The molecular basis of X-linked severe combined immunodeficiency: the role of the interleukin-2 receptor γ-chain as a common γ-chain, γ-c. Immunol. Rev. 138, 61–86 (1994).
Miyazaki, T. et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 266, 1045–1047 (1994).
Boussiotis, V. A. et al. Prevention of T cell anergy by signaling through the γ-c chain of the IL-2 receptor. Science 266, 1039–1042 (1994).
Russell, S. M. et al. Interaction of IL-2R β- and γ-c chains with Jak1 and Jak3: implications for XSCID and XCID. Science 266, 1042–1045 (1994). Important studies that established that JAK3 binds γc and predicted that mutations of JAK3 would also cause SCID.
Notarangelo, L. D. et al. Of genes and phenotypes: the immunological and molecular spectrum of combined immune deficiency. Defects of the γ(c)-JAK3 signaling pathway as a model. Immunol. Rev. 178, 39–48 (2000).
Buckley, R. H. et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J. Pediatr. 130, 378–387 (1997).
Buckley, R. H. Primary immunodeficiency diseases due to defects in lymphocytes. N. Engl. J. Med. 343, 1313–1324 (2000).
Candotti, F., Notarangelo, L., Visconti, R. & O'Shea, J. Molecular aspects of primary immunodeficiencies: lessons from cytokine and other signaling pathways. J. Clin. Invest. 109, 1261–1269 (2002).
Buckley, R. H. Advances in the understanding and treatment of human severe combined immunodeficiency. Immunol. Res. 22, 237–251 (2000).
Russell, S. M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995). The first evidence that mutations of JAK3 cause SCID, which established the crucial, yet highly selective, in vivo functions of JAK3.
Mella, P. et al. Eleven novel JAK3 mutations in patients with severe combined immunodeficiency-including the first patients with mutations in the kinase domain. Hum. Mutat. 18, 355–356 (2001).
Notarangelo, L. D. et al. Mutations in severe combined immune deficiency (SCID) due to JAK3 deficiency. Hum. Mutat. 18, 255–263 (2001).
Candotti, F. et al. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood 90, 3996–4003 (1997).
Roberts, J. L. et al. Janus kinase 3 (JAK3) deficiency: clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood 103, 2009–2018 (2004).
Puel, A., Ziegler, S. F., Buckley, R. H. & Leonard, W. J. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nature Genet. 20, 394–397 (1998).
Patel, D. D. et al. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N. Engl. J. Med. 342, 1325–1332 (2000).
Antoine, C. et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet 361, 553–560 (2003).
Haddad, E. et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood 91, 3646–3653 (1998).
Hacein-Bey-Abina, S. et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 346, 1185–1193 (2002).
Malek, T. R., Porter, B. O. & He, Y. W. Multiple γc-dependent cytokines regulate T-cell development. Immunol. Today 20, 71–76 (1999).
Peschon, J. J. et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180, 1955–1960 (1994).
Candeias, S., Peschon, J. J., Muegge, K. & Durum, S. K. Defective T-cell receptor-γ gene rearrangement in interleukin-7 receptor knockout mice. Immunol. Lett. 57, 9–14 (1997).
Fry, T. J. & Mackall, C. L. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 22, 564–571 (2001).
Fry, T. J. & Mackall, C. L. Interleukin-7: from bench to clinic. Blood 99, 3892–3904 (2002).
Maraskovsky, E. et al. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J. Immunol. 157, 5315–5323 (1996).
Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature Immunol. 1, 426–432 (2000).
Schluns, K. S., Williams, K., Ma, A., Zheng, X. X. & Lefrancois, L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168, 4827–4831 (2002).
Becker, T. C. et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195, 1541–1548 (2002).
Goldrath, A. W. et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195, 1515–1522 (2002).
Seddon, B., Tomlinson, P. & Zamoyska, R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nature Immunol. 4, 680–686 (2003).
Cooper, M. A. et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100, 3633–3638 (2002).
Fehniger, T. A., Cooper, M. A. & Caligiuri, M. A. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 13, 169–183 (2002).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002). An interesting study on the newest γc cytokine.
Nelson, B. H. Interleukin-2 signaling and the maintenance of self-tolerance. Curr. Dir. Autoimmun. 5, 92–112 (2002).
Refaeli, Y., Van Parijs, L. & Abbas, A. K. Genetic models of abnormal apoptosis in lymphocytes. Immunol. Rev. 169, 273–282 (1999).
Bassiri, H. & Carding, S. R. A requirement for IL-2/IL-2 receptor signaling in intrathymic negative selection. J. Immunol. 166, 5945–5954 (2001).
Shevach, E. M. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 18, 423–449 (2000).
Malek, T. R. The main function of IL-2 is to promote the development of T regulatory cells. J. Leukoc. Biol. 74, 961–965 (2003).
Frucht, D. M. et al. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun. 2, 422–432 (2001).
Druker, B. J. Perspectives on the development of a molecularly targeted agent. Cancer Cell 1, 31–36 (2002).
Cools, J. et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N. Engl. J. Med. 348, 1201–1214 (2003).
Goekjian, P. G. & Jirousek, M. R. Protein kinase C in the treatment of disease: signal transduction pathways, inhibitors, and agents in development. Curr. Med. Chem. 6, 877–903 (1999).
Goekjian, P. G. & Jirousek, M. R. Protein kinase C inhibitors as novel anticancer drugs. Expert Opin. Investig. Drugs 10, 2117–2140 (2001).
Duby, J. J., Campbell, R. K., Setter, S. M., White, J. R. & Rasmussen, K. A. Diabetic neuropathy: an intensive review. Am. J. Health Syst. Pharm. 61, 160–173 (2004).
Sudbeck, E. A. et al. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin. Cancer Res. 5, 1569–1582 (1999).
Saemann, M. D. et al. Suppression of early T-cell-receptor-triggered cellular activation by the Janus kinase 3 inhibitor WHI-P-154. Transplantation 75, 1864–1872 (2003).
McMillan, S. J., Bishop, B., Townsend, M. J., McKenzie, A. N. & Lloyd, C. M. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J. Exp. Med. 195, 51–57 (2002).
Malaviya, R., Zhu, D., Dibirdik, I. & Uckun, F. M. Targeting Janus kinase 3 in mast cells prevents immediate hypersensitivity reactions and anaphylaxis. J. Biol. Chem. 274, 27028–27038 (1999).
Malaviya, R., Navara, C. & Uckun, F. M. Role of Janus kinase 3 in mast cell-mediated innate immunity against Gram-negative bacteria. Immunity 15, 313–321 (2001).
Saemann, M. D. et al. CD40 triggered human monocyte-derived dendritic cells convert to tolerogenic dendritic cells when JAK3 activity is inhibited. Transplant. Proc. 34, 1407–1408 (2002).
Jabara, H. H. et al. Role of JAK3 in CD40-mediated signaling. Blood 92, 2435–2440 (1998).
Migone, T. S. et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 269, 79–81 (1995).
Malaviya, R. & Uckun, F. M. Genetic and biochemical evidence for a critical role of Janus kinase (JAK)-3 in mast cell-mediated type I hypersensitivity reactions. Biochem. Biophys. Res. Commun. 257, 807–813 (1999).
Thompson, J. E. et al. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg. Med. Chem. Lett. 12, 1219–1223 (2002).
Meydan, N. et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379, 645–648 (1996).
Kirken, R. A. et al. Tyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cells. J. Leukoc. Biol. 65, 891–899 (1999).
Parganas, E. et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93, 385–395 (1998).
Rodig, S. J. et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93, 373–383 (1998).
Changelian, P. S. et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302, 875–878 (2003). The first report of a selective potent JAK3 inhibitor with efficacy in a NHP model of transplantation.
Kudlacz, E. et al. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am. J. Transplant. 4, 51–57 (2004).
Waldmann, T. A. & O'Shea, J. The use of antibodies against the IL-2 receptor in transplantation. Curr. Opin. Immunol. 10, 507–512 (1998).
Townsend, J. M. et al. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13, 573–583 (2000).
Temann, U. A., Ray, P. & Flavell, R. A. Pulmonary overexpression of IL-9 induces TH2 cytokine expression, leading to immune pathology. J. Clin. Invest. 109, 29–39 (2002).
Saemann, M. D. et al. Prevention of CD40-triggered dendritic cell maturation and induction of T-cell hyporeactivity by targeting of Janus kinase 3. Am. J. Transplant. 3, 1341–1349 (2003).
Hanissian, S. H. & Geha, R. S. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity 6, 379–387 (1997).
Tibbles, H. E. et al. Role of a JAK3-dependent biochemical signaling pathway in platelet activation and aggregation. J. Biol. Chem. 276, 17815–17822 (2001).
Narla, R. K., Liu, X. P., Klis, D. & Uckun, F. M. Inhibition of human glioblastoma cell adhesion and invasion by 4-(4′-hydroxylphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P131) and 4-(3′-bromo-4′-hydroxylphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P154). Clin. Cancer Res. 4, 2463–2471 (1998).
Shimoda, K. et al. Tyk2 plays a restricted role in IFNα signaling, although it is required for IL-12-mediated T cell function. Immunity 13, 561–571 (2000).
Karaghiosoff, M. et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity 13, 549–560 (2000).
Schleicher, U. et al. Control of Leishmania major in the absence of Tyk2 kinase. Eur. J. Immunol. 34, 519–529 (2004).
Trinchieri, G., Pflanz, S. & Kastelein, R. A. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19, 641–644 (2003). An excellent review of the small family of dimeric immunoregulatory cytokines.
Parham, C. et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168, 5699–5708 (2002).
Ortmann, R., Smeltz, R., Yap, G., Sher, A. & Shevach, E. M. A heritable defect in IL-12 signaling in B10.Q/J mice. I. In vitro analysis. J. Immunol. 166, 5712–5719 (2001).
Yap, G. S., Ortmann, R., Shevach, E. & Sher, A. A heritable defect in IL-12 signaling in B10.Q/J mice. II. Effect on acute resistance to Toxoplasma gondii and rescue by IL-18 treatment. J. Immunol. 166, 5720–5725 (2001).
Shaw, M. H. et al. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc. Natl Acad. Sci. USA 100, 11594–11599 (2003). An interesting study showing that the inhibition of TYK2 attenuates the development of arthritis.
Greenlund, A. C., Farrar, M. A., Viviano, B. L. & Schreiber, R. D. Ligand-induced IFNγ receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J. 13, 1591–1600 (1994).
Schindler, U., Wu, P., Rothe, M., Brasseur, M. & McKnight, S. L. Components of a Stat recognition code: evidence for two layers of molecular selectivity. Immunity 2, 689–697 (1995).
Yu, H. & Jove, R. The STATs of cancer: new molecular targets come of age. Nature Rev. Cancer 4, 97–105 (2004). An excellent review of the role of STAT activation in cancer and strategies for targeting STATs.
Turkson, J. et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol. Cancer Ther. 3, 261–269 (2004).
Darnell, J. E. Jr. Transcription factors as targets for cancer therapy. Nature Rev. Cancer 2, 740–749 (2002).
Wang, T. et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nature Med. 10, 48–54 (2004).
Wurster, A. L., Tanaka, T. & Grusby, M. J. The biology of Stat4 and Stat6. Oncogene 19, 2577–2584 (2000).
Youssef, S. et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a TH2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420, 78–84 (2002).
Leung, B. P. et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J. Immunol. 170, 1524–1530 (2003).
Nath, N., Giri, S., Prasad, R., Singh, A. K. & Singh, I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor for multiple sclerosis therapy. J. Immunol. 172, 1273–1286 (2004).
Chiang, P. H. et al. Mechanistic insights into impaired dendritic cell function by rapamycin: inhibition of Jak2/Stat4 signaling pathway. J. Immunol. 172, 1355–1363 (2004).
Coon, M. E., Diegel, M., Leshinsky, N. & Klaus, S. J. Selective pharmacologic inhibition of murine and human IL-12-dependent TH1 differentiation and IL-12 signaling. J. Immunol. 163, 6567–6574 (1999).
Bright, J. J., Du, C., Coon, M., Sriram, S. & Klaus, S. J. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated TH1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. J. Immunol. 161, 7015–7022 (1998).
Yang, Z. et al. Inhibition of STAT4 activation by lisofylline is associated with the protection of autoimmune diabetes. Ann. NY Acad. Sci. 1005, 409–411 (2003).
Levy, D. E. & Darnell, J. E. Jr. Stats: transcriptional control and biological impact. Nature Rev. Mol. Cell Biol. 3, 651–662 (2002).
Dupuis, S. et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nature Genet. 33, 388–391 (2003).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 3, 991–998 (2002).
Park, C., Li, S., Cha, E. & Schindler, C. Immune response in Stat2 knockout mice. Immunity 13, 795–804 (2000).
Wormald, S. & Hilton, D. J. Inhibitors of cytokine signal transduction. J. Biol. Chem. 279, 821–824 (2004).
Turnley, A. M., Faux, C. H., Rietze, R. L., Coonan, J. R. & Bartlett, P. F. Suppressor of cytokine signaling 2 regulates neuronal differentiation by inhibiting growth hormone signaling. Nature Neurosci. 5, 1155–1162 (2002).
Egwuagu, C. E. et al. Suppressors of cytokine signaling proteins are differentially expressed in TH1 and TH2 cells: implications for TH cell lineage commitment and maintenance. J. Immunol. 168, 3181–3187 (2002).
Seki, Y. et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nature Med. 9, 1047–1054 (2003).
Kirken, R. A. et al. Tyrphostin AG490 selectively inhibits activation of the JAK3/STAT5/MAPK pathway and rejection of rat heart allografts. Transplant. Proc. 33, 95 (2001).
Behbod, F. et al. Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J. Immunol. 166, 3724–3732 (2001).
Wang, L. H. et al. Selective disruption of interleukin 4 autocrine-regulated loop by a tyrosine kinase inhibitor restricts activity of T-helper 2 cells. Blood 95, 3816–3822 (2000).
Kirken, R. A. et al. Functional uncoupling of the Janus kinase 3-Stat5 pathway in malignant growth of human T cell leukemia virus type 1-transformed human T cells. J. Immunol. 165, 5097–5104 (2000).
Amin, H. M. et al. Inhibition of JAK3 induces apoptosis and decreases anaplastic lymphoma kinase activity in anaplastic large cell lymphoma. Oncogene 22, 5399–5407 (2003).
Stepkowski, S. M. et al. Allochimeric class I MHC protein-induced tolerance by partial TCR engagement requires activation of both CTL4- and common γ-chain-dependent cytokine signals. Transplantation 80, 1227–1235 (2002).
Cetkovic-Cvrlje, M., Dragt, A. L. & Uckun, F. M. Prevention of islet allograft rejection in diabetic mice by targeting Janus Kinase 3 with 4-(4′-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline (JANEX-1). Arzneimittelforschung 53, 648–654 (2003).
Cetkovic-Cvrlje, M., Dragt, A. L., Vassilev, A., Liu, X. P. & Uckun, F. M. Targeting JAK3 with JANEX-1 for prevention of autoimmune type 1 diabetes in NOD mice. Clin. Immunol. 106, 213–225 (2003).
Cetkovic-Cvrlje, M., Roers, B. A., Waurzyniak, B., Liu, X. P. & Uckun, F. M. Targeting Janus kinase 3 to attenuate the severity of acute graft-versus-host disease across the major histocompatibility barrier in mice. Blood 98, 1607–1613 (2001).
Cetkovic-Cvrlje, M. et al. Treatment of post-bone marrow transplant acute graft-versus-host disease with a rationally designed JAK3 inhibitor. Leuk. Lymphoma 43, 1447–1453 (2002).
Uckun, F. M., Roers, B. A., Waurzyniak, B., Liu, X. P. & Cetkovic-Cvrlje, M. Janus kinase 3 inhibitor WHI-P131/ JANEX-1 prevents graft-versus-host disease but spares the graft-versus-leukemia function of the bone marrow allografts in a murine bone marrow transplantation model. Blood 99, 4192–4199 (2002).
Trieu, V. N., Liu, R., Liu, X. P. & Uckun, F. M. A specific inhibitor of Janus kinase-3 increases survival in a transgenic mouse model of amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 267, 22–25 (2000).
Barata, J. T. et al. IL-7-dependent human leukemia T-cell line as a valuable tool for drug discovery in T-ALL. Blood 103, 1891–1900 (2004).
Stepkowski, S. M. et al. PNU156804 inhibits Jak3 tyrosine kinase and rat heart allograft rejection. Transplant. Proc. 33, 3272–3280 (2001).
Stepkowski, S. M. et al. Selective inhibitor of Janus tyrosine kinase 3, PNU156804, prolongs allograft survival and acts synergistically with cyclosporine but additively with rapamycin. Blood 99, 680–689 (2002).
Wasik, M. A., Nowak, I., Zhang, Q. & Shaw, L. M. Suppression of proliferation and phosphorylation of Jak3 and STAT5 in malignant T-cell lymphoma cells by derivatives of octylamino-undecyl-dimethylxanthine. Leuk. Lymphoma 28, 551–560 (1998).
Siemasko, K. et al. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J. Immunol. 160, 1581–1588 (1998).
Elder, R. T. et al. The immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanisms. J. Immunol. 159, 22–27 (1997).
Manna, S. K. & Aggarwal, B. B. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-κB activation and gene expression. J. Immunol. 162, 2095–2102 (1999).
Brown, G. R. et al. Naphthyl ketones: a new class of Janus kinase 3 inhibitors. Bioorg. Med. Chem. Lett. 10, 575–579 (2000).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Dr O'Shea's and Borie's laboratory have received support from Pfizer. Dr Borie also has personal financial interests. Dr Changelian is employed by Pfizer and has personal financial interests.
Related links
Related links
DATABASES
LocusLink
Online Mendelian Inheritance in Man
hyperimmunoglobulin E syndrome
FURTHER INFORMATION
Transplant Immunology in the Department of Cardiothoracic Surgery, Stanford University
Glossary
- IMMUNOSUPPRESSANT
-
A compound that inhibits immune responses and is used to treat a wide range of diseases, from allergies and asthma to severe autoimmune disease and transplant rejection.
- PROTEIN TYROSINE KINASE
-
An enzyme with phosphotransferase activity, which specifically catalyses the transfer of phosphate from ATP to tyrosine residues on proteins.
- CYTOKINES
-
A large diverse group of small secreted glycoproteins, which regulate the function of a wide range of cells.
- HOMEOSTASIS
-
A generic term describing the status of physiological normality. With respect to the immune system, the concept refers to the maintenance of normal numbers and composition of peripheral lymphocytes.
- HELPER T CELLS
-
(TH). A subset of CD4+ T cells. Following antigen-mediated activation, these cells coordinate the immune response by the secretion of cytokines and cell–cell interactions.
- MEMORY T CELL
-
A long-lived T lymphocyte that has encountered its antigen. During subsequent encounters with the antigen, memory cells are more readily activated than naive cells.
- INTERLEUKIN
-
(IL). This is a generic term for cytokines that are produced by leukocytes, which have important roles in immunoregulation, host defence and inflammation.
- SELF TOLERANCE
-
The lack of response to self antigens; the concept refers to mechanisms that prevent the generation of immune and inflammatory responses to normal cells and tissues. Cytokines, such as interleukin-12, are one mechanism that controls tolerance.
- REGULATORY T CELL
-
(TReg). A lymphoid cell that suppresses humoral and cell-mediated immunity.
- DENDRITIC CELLS
-
(DCs). These professional antigen-presenting cells are increasingly recognized as having crucial immunoregulatory functions. They are found in various tissues where they take up antigens, process them, migrate to the lymph nodes and present the antigens to T cells.
- MAST CELL
-
A bone marrow-derived cell that is present in various tissues; they are important contributors to allergic disease and recent data have also pointed towards a role in arthritis.
Rights and permissions
About this article
Cite this article
O'Shea, J., Pesu, M., Borie, D. et al. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov 3, 555–564 (2004). https://doi.org/10.1038/nrd1441
Issue Date:
DOI: https://doi.org/10.1038/nrd1441
This article is cited by
-
Medicinal chemistry perspective of JAK inhibitors: synthesis, biological profile, selectivity, and structure activity relationship
Molecular Diversity (2024)
-
Tofacitinib fails to prevent T cell transfer colitis in mice but ameliorates disease activity
Scientific Reports (2023)
-
Janus Kinase Inhibitors and Non-Melanoma Skin Cancer
Current Treatment Options in Oncology (2021)
-
MC-LR-induced interaction between M2 macrophage and biliary epithelial cell promotes biliary epithelial cell proliferation and migration through regulating STAT3
Cell Biology and Toxicology (2021)
-
JAK/STAT pathway and molecular mechanism in bone remodeling
Molecular Biology Reports (2020)