Abstract
Interleukin-6 (IL-6) is a pivotal cytokine with a diverse repertoire of physiological functions that include regulation of immune cell proliferation and differentiation. Dysregulation of IL-6 signalling is associated with inflammatory and lymphoproliferative disorders such as rheumatoid arthritis and Castleman disease, and several classes of therapeutics have been developed that target components of the IL-6 signalling pathway. So far, monoclonal antibodies against IL-6 or IL-6 receptor (IL-6R) and Janus kinases (JAK) inhibitors have been successfully developed for the treatment of autoimmune diseases such as rheumatoid arthritis. However, clinical trials of agents targeting IL-6 signalling have also raised questions about the diseases and patient populations for which such agents have an appropriate benefit–risk profile. Knowledge from clinical trials and advances in our understanding of the complexities of IL-6 signalling, including the potential to target an IL-6 trans-signalling pathway, are now indicating novel opportunities for therapeutic intervention. In this Review, we overview the roles of IL-6 in health and disease and analyse progress with several approaches of inhibiting IL-6-signalling, with the aim of illuminating when and how to apply IL-6 blockade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Elliott, M. J. et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 344, 1105–1110 (1994).
Grau, G. E. & Maennel, D. N. TNF inhibition and sepsis — sounding a cautionary note. Nat. Med. 3, 1193–1195 (1997).
Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2, 364–371 (2002).
Aggarwal, B. B., Gupta, S. C. & Kim, J. H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 119, 651–665 (2012).
Lu, Z. Y. et al. High amounts of circulating interleukin (IL)-6 in the form of monomeric immune complexes during anti-IL-6 therapy. Towards a new methodology for measuring overall cytokine production in human in vivo. Eur. J. Immunol. 22, 2819–2824 (1992).
Alonzi, T. et al. Interleukin 6 is required for the development of collagen-induced arthritis. J. Exp. Med. 187, 461–468 (1998).
Ohshima, S. et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc. Natl Acad. Sci. USA 95, 8222–8226 (1998).
Tanaka, T., Narazaki, M. & Kishimoto, T. Therapeutic targeting of the interleukin-6 receptor. Annu. Rev. Pharmacol. Toxicol. 52, 199–219 (2012).
Tanaka, T., Narazaki, M. & Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 (2014).
Reynolds, A., Koenig, A. S., Bananis, E. & Singh, A. When is switching warranted among biologic therapies in rheumatoid arthritis? Expert Rev. Pharmacoecon. Outcomes Res. 12, 319–333 (2012).
Rose-John, S., Winthrop, K. & Calabrese, L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat. Rev. Rheumatol. 13, 399–409 (2017).
Bingham, C. O. 3rd et al. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Ann. Rheum. Dis. 74, 818–822 (2015).
Kishimoto, T. IL-6: from its discovery to clinical applications. Int. Immunol. 22, 347–352 (2010). This is an impressive summary of the history of the discovery of IL-6, IL-6R, gp130, STAT3 and SOCS3 by Kishimoto and the development of the antibody tocilizumab, which is now approved in more than 100 countries for the treatment of autoimmune diseases.
Heinrich, P. C., Castell, J. V. & Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 265, 621–636 (1990).
Cressman, D. E. et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274, 1379–1383 (1996).
Calabrese, L. H. & Rose-John, S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol. 10, 720–727 (2014).
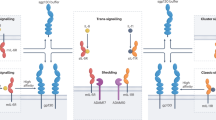
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 (2011). This article summarizes the evidence that IL-6 trans -signalling via the soluble IL-6R is mainly pro-inflammatory signalling, whereas signalling via the membrane-bound IL-6R is rather regenerative and protective.
Waage, A., Brandtzaeg, P., Halstensen, A., Kierulf, P. & Espevik, T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 169, 333–338 (1989).
Campbell, I. L. et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl Acad. Sci. USA 90, 10061–10065 (1993).
Schaper, F. & Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 26, 475–487 (2015).
Rose-John, S. & Heinrich, P. C. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem. J. 300, 281–290 (1994). In this article, the paradigm of IL-6 trans -signalling for IL-6 activities via sIL-6R is used for the first time.
Rose-John, S., Scheller, J. & Schaper, F. “Family reunion” — a structured view on the composition of the receptor complexes of interleukin-6-type and interleukin-12-type cytokines. Cytokine Growth Factor Rev. 26, 471–474 (2015).
Garbers, C. et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 23, 85–97 (2012).
Riethmueller, S. et al. Proteolytic origin of the soluble human IL-6R in vivo and a decisive role of N-glycosylation. PLoS Biol. 15, e2000080 (2017). This is the first direct demonstration that sIL-6R found in human serum is generated by limited proteolysis.
Lust, J. et al. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine 4, 96–100 (1992).
Jostock, T. et al. Soluble gp130 is the natural inhibitor of soluble IL-6R transsignaling responses. Eur. J. Biochem. 268, 160–167 (2001). This paper demonstrates that the soluble form of gp130, which is found at high concentration in human serum, specifically neutralizes IL-6 trans -signalling via sIL-6R.
Jones, S. A., Scheller, J. & Rose-John, S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 121, 3375–3383 (2011).
Rafiq, S. et al. A common variant of the interleukin 6 receptor (IL-6r) gene increases IL-6r and IL-6 levels, without other inflammatory effects. Genes Immun. 8, 552–559 (2007).
Garbers, C. et al. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim. Biophys. Acta 1842, 1485–1494 (2014).
Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 379, 1214–1224 (2012).
IL6R Genetics Consortium Emerging Risk Factors Collaboration et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 379, 1205–1213 (2012).
Scheller, J. & Rose-John, S. The interleukin 6 pathway and atherosclerosis. Lancet 380, 338 (2012).
Heink, S. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 18, 74–85 (2017). This study identifies IL-6 trans -presentation as a signalling mode of IL-6 essential during the priming of pathogenic T H 17 cells in autoimmune disease of the central nervous system.
Carpenter, R. L. & Lo, H. W. STAT3 target genes relevant to human cancers. Cancers 6, 897–925 (2014).
Rebouissou, S. et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 457, 200–204 (2009). This study is the first to report somatic activating mutations in gp130, which are associated with liver inflammatory adenomas and hepatocellular tumours.
Taniguchi, K. et al. YAP-IL-6ST autoregulatory loop activated on APC loss controls colonic tumorigenesis. Proc. Natl Acad. Sci. USA 114, 1643–1648 (2017).
Taniguchi, K. et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519, 57–62 (2015). This study describes gp130–SRC–YAP signalling, which is independent of STAT3 activation, in regeneration and inflammation in the intestine and in the liver.
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Carey, A. L. et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697 (2006).
Steensberg, A. et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 529, 237–242 (2000).
Strang, A. C. et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 229, 174–181 (2013).
Tournadre, A. et al. Management of dyslipidaemia in high-risk patients with recent-onset rheumatoid arthritis: targets still not met despite specific recommendations. Results from the ESPOIR cohort during the first five years of follow-up. Clin. Exp. Rheumatol. 35, 296–302 (2017).
Bastard, J. P. et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 85, 3338–3342 (2000).
Nicklas, B. J., You, T. & Pahor, M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ 172, 1199–1209 (2005).
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E. & Ridker, P. M. C-Reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286, 327–334 (2001).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Carey, A. L. et al. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with Type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia 47, 1029–1037 (2004).
Wallenius, V. et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8, 75–79 (2002). This is the first study to report mature-onset obesity in mice lacking a functional IL-6 gene.
Wunderlich, T. F. et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12, 237–249 (2010).
Xu, E. et al. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat. Commun. 8, 14803 (2017).
Matthews, V. B., Allen, T. L., Risis, S. & Chan, M. H. S. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53, 2431–2441 (2010).
Kraakman, M. J. et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 21, 403–416 (2015). This paper provides evidence that IL-6 trans -signalling via sIL-6R is responsible for high-fat diet-induced recruitment of macrophages into adipose tissue.
Braune, J. et al. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J. Immunol. 198, 2927–2934 (2017).
Mauer, J. et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15, 423–430 (2014). This study provides compelling mechanistic evidence that IL-6 drives alternative differentiation of macrophages by inducing the expression of the IL-4R and responsiveness to IL-4.
Muller, N. et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J. Lipid Res. 56, 1034–1042 (2015).
Kraakman, M. J. et al. Targeting gp130 to prevent inflammation and promote insulin action. Diabetes Obes. Metab. 15 (Suppl. 3), 170–175 (2013).
Nishimoto, N. et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 106, 2627–2632 (2005).
Cheung, C.-L. L., Xiao, S.-M. M. & Kung, A. W. Genetic epidemiology of age-related osteoporosis and its clinical applications. Nat. Rev. Rheumatol. 6, 507–517 (2010).
De Benedetti, F. et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 54, 3551–3563 (2006).
Poli, V. et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 13, 1189–1196 (1994).
Carlsten, H. Immune responses and bone loss: the estrogen connection. Immunol. Rev. 208, 194–206 (2005).
Udagawa, N. et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J. Exp. Med. 182, 1461–1468 (1995).
Prystaz, K. et al. Distinct effects of interleukin-6 classic and trans-signaling in bone fracture healing. Am. J. Pathol. 188, 474–490 (2018).
Edwards, C. J. & Williams, E. The role of interleukin-6 in rheumatoid arthritis-associated osteoporosis. Osteoporos. Int. 21, 1287–1293 (2010).
van Staa, T. P., Geusens, P., Bijlsma, J. W., Leufkens, H. G. & Cooper, C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 54, 3104–3112 (2006).
Kume, K., Amano, K., Yamada, S. & Kanazawa, T. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology 53, 900–903 (2014).
Cray, C., Zaias, J. & Altman, N. H. Acute phase response in animals: a review. Comp. Med. 59, 517–526 (2009).
Gauldie, J., Richards, C., Harnish, D., Lansdorp, P. & Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl Acad. Sci. USA 84, 7251–7255 (1987). This is the first report to describe the identity of hepatocyte-stimulating factor with IL-6.
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Hoge, J. et al. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J. Immunol. 190, 703–711 (2013).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Lang, V. R. et al. Risk of infections in rheumatoid arthritis patients treated with tocilizumab. Rheumatology 51, 852–857 (2012).
Gabay, C. et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550 (2013).
Burmester, G. R. & Pope, J. E. Novel treatment strategies in rheumatoid arthritis. Lancet 389, 2338–2348 (2017).
Suthaus, J. et al. HHV-8-encoded viral IL-6 collaborates with mouse IL-6 in the development of multicentric Castleman disease in mice. Blood 119, 5173–5181 (2012).
Song, S. N. et al. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood 116, 3627–3634 (2010).
Liu, A. Y. et al. Idiopathic multicentric Castleman's disease: a systematic literature review. Lancet Haematol. 3, e163–e175 (2016).
Villiger, P. M. et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 387, 1921–1927 (2016).
Stone, J. H. et al. Trial of tocilizumab in giant-cell arteritis. N. Engl. J. Med. 377, 317–328 (2017). This study shows the efficacy of subcutaneous anti-IL-6R antibody administration in the management of giant cell arteritis, which enables avoidance of long-term corticoid treatment for this disease.
Chihara, N. et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc. Natl Acad. Sci. USA 108, 3701–3706 (2011).
Araki, M. et al. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology 82, 1302–1306 (2014). This is a case series that suggests that tocilizumab is an efficient treatment for NMO, an autoimmune disease of the central nervous system that is mediated by antibodies to the water channel AQP4.
Kleiter, I. & Gold, R. Present and future therapies in neuromyelitis optica spectrum disorders. Neurotherapeutics 13, 70–83 (2016).
Diehl, S. et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13, 805–815 (2000).
Diehl, S. & Rincon, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 39, 531–536 (2002).
Rincon, M., Anguita, J., Nakamura, T., Fikrig, E. & Flavell, R. A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185, 461–469 (1997).
Mendel, I., Katz, A., Kozak, N., Ben-Nun, A. & Revel, M. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur. J. Immunol. 28, 1727–1737 (1998).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006). This is the first paper to show that IL-6 is a differentiation factor of T H 17 cells.
Mangan, P. R. et al. Transforming growth factor-beta induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). This is a key study to suggest that IL-6 may dictate whether naive T cells differentiate into pro-inflammatory T H 17 cells or FOXP3+ T reg cells during antigen-specific priming of T cells in the peripheral immune compartment.
Zhou, L. et al. TGF-beta-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240 (2008).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Kreymborg, K. et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179, 8098–8104 (2007).
Eyerich, S. et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 119, 3573–3585 (2009).
Trifari, S., Kaplan, C. D., Tran, E. H., Crellin, N. K. & Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 10, 864–871 (2009).
Duhen, T., Geiger, R., Jarrossay, D., Lanzavecchia, A. & Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10, 857–863 (2009). This is a description of T H 22 cells as a subset of skin-homing T H cells that produce IL-22 and are induced in response to a combination of IL-6 and TNF.
Moyat, M. et al. IL-22-induced antimicrobial peptides are key determinants of mucosal vaccine-induced protection against H. pylori in mice. Mucosal Immunol. 10, 271–281 (2017).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012).
Fischer, M. et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat. Biotechnol. 15, 142–145 (1997). This is the first description of the designer cytokine hyper-IL-6 consisting of sIL-6R covalently linked to IL-6, which is widely used as a mimic of IL-6 trans -signalling.
Briso, E., Dienz, O. & Rincon, M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J. Immunol. 180, 7102–7106 (2008).
Jones, G. W. et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J. Immunol. 184, 2130–2139 (2010).
Scheller, J., Chalaris, A., Garbers, C. & Rose-John, S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 32, 380–387 (2011).
Hirota, K. et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204, 41–47 (2007).
Michel, M. L. et al. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc. Natl Acad. Sci. USA 105, 19845–19850 (2008).
Lochner, M. et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J. Exp. Med. 205, 1381–1393 (2008).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009). This is a demonstration of regenerative and anti-inflammatory properties of IL-6 in intestinal regeneration.
Tsantikos, E. et al. Autoimmune disease in Lyn-deficient mice is dependent on an inflammatory environment established by IL-6. J. Immunol. 184, 1348–1360 (2010).
Tsantikos, E. et al. Interleukin-6 trans-signaling exacerbates inflammation and renal pathology in lupus-prone mice. Arthritis Rheum. 65, 2691–2702 (2013).
Happel, K. I. et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202, 761–769 (2005).
Igyarto, B. Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).
Vinuesa, C. G., Linterman, M. A., Yu, D. & MacLennan, I. C. Follicular helper T cells. Annu. Rev. Immunol. 34, 335–368 (2016).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Karnowski, A. et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J. Exp. Med. 209, 2049–2064 (2012).
Chavele, K. M., Merry, E. & Ehrenstein, M. R. Cutting edge: circulating plasmablasts induce the differentiation of human T follicular helper cells via IL-6 production. J. Immunol. 194, 2482–2485 (2015).
Harker, J. A., Lewis, G. M., Mack, L. & Zuniga, E. I. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829 (2011). In this study, IL-6 produced by FDCs is identified as indispensable for the generation of T FH cells, the generation of neutralizing antibodies and virus control in late phases of chronic viral infection.
Nakayamada, S. et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35, 919–931 (2011).
Nakayamada, S. et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J. Immunol. 192, 2156–2166 (2014).
Choi, Y. S., Eto, D., Yang, J. A., Lao, C. & Crotty, S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190, 3049–3053 (2013).
Schooltink, H. & Rose-John, S. Cytokines as therapeutic drugs. J. Interferon Cytokine Res. 22, 505–516 (2002).
Boulanger, M. J., Chow, D. C., Brevnova, E. E. & Garcia, K. C. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science 300, 2101–2104 (2003).
Skiniotis, G., Boulanger, M. J., Garcia, K. C. & Walz, T. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat. Struct. Mol. Biol. 12, 545–551 (2005).
van Rhee, F. et al. Siltuximab for multicentric Castleman's disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 15, 966–974 (2014).
Aletaha, D. et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet 389, 1206–1217 (2017).
Mease, P. et al. A phase II, double-blind, randomised, placebo-controlled study of BMS945429 (ALD518) in patients with rheumatoid arthritis with an inadequate response to methotrexate. Ann. Rheum. Dis. 71, 1183–1189 (2012).
Weinblatt, M. E. et al. The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase Iib, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol. 67, 2591–2600 (2015).
Mease, P. J. et al. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase Iib study of adults with active psoriatic arthritis. Arthritis Rheumatol. 68, 2163–2173 (2016).
Shaw, S. et al. Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. mAbs 6, 774–782 (2014).
Kretsos, K. et al. Safety and pharmacokinetics of olokizumab, an anti-IL-6 monoclonal antibody, administered to healthy male volunteers: A randomized phase I study. Clin. Pharmacol. Drug Dev. 3, 388–395 (2014).
Genovese, M. C. et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised phase Iib study. Ann. Rheum. Dis. 73, 1607–1615 (2014).
Klein, B., Lu, Z. Y., Gaillard, J. P., Harousseau, J. L. & Bataille, R. Inhibiting IL-6 in human multiple myeloma. Curr. Top. Microbiol. Immunol. 182, 237–244 (1992).
Klein, B. et al. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood 78, 1198–1204 (1991).
Nishimoto, N. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112, 3959–3964 (2008).
Moots, R. J. et al. Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology 56, 541–549 (2017).
Schuster, B. et al. Signaling of human ciliary neurotrophic factor (CNTF) revisited. The interleukin-6 receptor can serve as an alpha-receptor for CTNF. J. Biol. Chem. 278, 9528–9535 (2003).
Garbers, C. et al. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 Protein receptor homodimer. J. Biol. Chem. 288, 4346–4354 (2013).
Van Roy, M. et al. The preclinical pharmacology of the high affinity anti-IL-6R Nanobody(R) ALX-0061 supports its clinical development in rheumatoid arthritis. Arthritis Res. Ther. 17, 135 (2015).
Lacroix, M. et al. Novel insights into interleukin 6 (IL-6) cis- and trans-signaling pathways by differentially manipulating the assembly of the IL-6 signaling complex. J. Biol. Chem. 290, 26943–26953 (2015).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl Med. 6, 224ra25 (2014).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000).
Rabe, B. et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood 111, 1021–1028 (2008).
Lesina, M. et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19, 456–469 (2011).
Zhang, H. et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J. Clin. Invest. 123, 1019–1031 (2013).
Bergmann, J. et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology 65, 89–103 (2017).
Sodenkamp, J. et al. Therapeutic targeting of interleukin-6 trans-signaling does not affect the outcome of experimental tuberculosis. Immunobiology 217, 996–1004 (2012).
German Clinical Trials Register. A multi-centre, exploratory trial to assess the mechanisms of molecular activity, safety and tolerability of one dose level of FE 999301 by intravenous infusions in patients with active inflammatory bowel disease (IBD). http://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00010101 German Clinical Trials Register (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03235752 (2018).
Taga, T. et al. Functional inhibition of hematopoietic and neurotrophic cytokines by blocking the interleukin 6 signal transducer gp130. Proc. Natl Acad. Sci. USA 89, 10998–11001 (1992).
Gu, Z. J. et al. Anti-gp130 transducer monoclonal antibodies specifically inhibiting ciliary neurotrophic factor, interleukin-6, interleukin-11, leukemia inhibitory factor or oncostatin M. J. Immunol. Methods 190, 21–27 (1996).
Yoshida, K., Taga, T. & Saito, M. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc. Natl Acad. Sci. USA 93, 407–411 (1996).
Hirota, H. et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97, 189–198 (1999).
Stark, G. & Darnell, J. The JAK-STAT pathway at twenty. Immunity 36, 503–514 (2012).
O'Shea, J. & Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36, 542–550 (2012).
Yamaoka, K. et al. The Janus kinases (Jaks). Genome Biol. 5, 253 (2004).
Stahl, N. et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science 263, 92–95 (1994).
Guschin, D. et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 14, 1421–1429 (1995).
Rodig, S. J. et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93, 373–383 (1998).
Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
James, C. et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148 (2005).
Levine, R. L. et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7, 387–397 (2005).
Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790 (2005).
Meyer, S. C. & Levine, R. L. Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin. Cancer Res. 20, 2051–2059 (2014).
Ghoreschi, K. et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J. Immunol. 186, 4234–4243 (2011).
Winthrop, K. L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 13, 320 (2017).
O'Shea, J., Kontzias, A., Yamaoka, K., Tanaka, Y. & Laurence, A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 72 (Suppl. 2), ii111–ii115 (2013).
Fleischmann, R. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367, 495–507 (2012).
van Vollenhoven, R. F. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 367, 508–519 (2012).
Fleischmann, R. et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390, 457–468 (2017).
Dougados, M. et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann. Rheum. Dis. 76, 88–95 (2017).
Fleischmann, R. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 69, 506–517 (2017).
Taylor, P. C. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376, 652–662 (2017).
Schwartz, D. M. et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 17, 78 (2017).
Wu, P., Nielsen, T. E. & Clausen, M. H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 36, 422–439 (2015).
Chrencik, J. E. et al. Structural and thermodynamic characterization of the TYK2 and JAK3 kinase domains in complex with CP-690550 and CMP-6. J. Mol. Biol. 400, 413–433 (2010).
Williams, N. K. et al. Dissecting specificity in the Janus kinases: the structures of JAK-specific inhibitors complexed to the JAK1 and JAK2 protein tyrosine kinase domains. J. Mol. Biol. 387, 219–232 (2009).
Clark, J. D., Flanagan, M. E. & Telliez, J. B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 57, 5023–5038 (2014).
Genovese, M. C. et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 68, 2857–2866 (2016).
Van Rompaey, L. et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J. Immunol. 191, 3568–3577 (2013).
Vermeire, S. et al. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389, 266–275 (2017).
Kremer, J. M. et al. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 68, 2867–2877 (2016).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02365649 (2017).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02780167 (2017).
Miklossy, G., Hilliard, T. S. & Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 12, 611–629 (2013).
Schust, J., Sperl, B., Hollis, A., Mayer, T. U. & Berg, T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 13, 1235–1242 (2006).
Pan, Y., Zhou, F., Zhang, R. & Claret, F. X. Stat3 inhibitor stattic exhibits potent antitumor activity and induces chemo- and radio-sensitivity in nasopharyngeal carcinoma. PLoS ONE 8, e54565 (2013).
O'Shea, J. J., Gadina, M. & Schreiber, R. D. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109 (Suppl.), S121–S131 (2002).
Song, H., Wang, R., Wang, S. & Lin, J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc. Natl Acad. Sci. USA 102, 4700–4705 (2005).
Kortylewski, M. et al. TLR agonist–Stat3 siRNA conjugates: cell-specific gene silencing and enhanced antitumor immune responses. Nat. Biotechnol. 27, 925–932 (2009).
Kortylewski, M. & Moreira, D. Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities. Cancer Immunol. Immunother. 66, 979–988 (2017).
Yoshizaki, K. et al. Isolation and characterization of B cell differentiation factor (BCDF) secreted from a human B lymphoblastoid cell line. J. Immunol. 132, 2948–2954 (1984).
Hirano, T. et al. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc. Natl Acad. Sci. USA 82, 5490–5494 (1985).
Hirano, T. et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324, 73–76 (1986). This is a landmark paper that first reported the cDNA sequence and basic characterization of IL-6.
Van Damme, J., Cayphas, S., Opdenakker, G., Billiau, A. & Van Snick, J. Interleukin 1 and poly(rI).poly(rC) induce production of a hybridoma growth factor by human fibroblasts. Eur. J. Immunol. 17, 1–7 (1987).
Jego, G., Bataille, R. & Pellat-Deceunynck, C. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood 97, 1817–1822 (2001).
Jego, G. et al. Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells. Blood 94, 701–712 (1999).
van Zaanen, H. C. et al. Endogenous interleukin 6 production in multiple myeloma patients treated with chimeric monoclonal anti-IL6 antibodies indicates the existence of a positive feed-back loop. J. Clin. Invest. 98, 1441–1448 (1996).
Belnoue, E. et al. Homing and adhesion patterns determine the cellular composition of the bone marrow plasma cell niche. J. Immunol. 188, 1283–1291 (2012).
Rodriguez-Bayona, B., Ramos-Amaya, A., Lopez-Blanco, R., Campos-Caro, A. & Brieva, J. A. STAT-3 activation by differential cytokines is critical for human in vivo-generated plasma cell survival and Ig secretion. J. Immunol. 191, 4996–5004 (2013).
Shapiro-Shelef, M. & Calame, K. Regulation of plasma-cell development. Nat. Rev. Immunol. 5, 230–242 (2005).
Shapiro-Shelef, M., Lin, K. I., Savitsky, D., Liao, J. & Calame, K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J. Exp. Med. 202, 1471–1476 (2005).
Shaffer, A. L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004).
Mesin, L., Di Niro, R., Thompson, K. M., Lundin, K. E. & Sollid, L. M. Long-lived plasma cells from human small intestine biopsies secrete immunoglobulins for many weeks in vitro. J. Immunol. 187, 2867–2874 (2011).
Yan, Y., Wang, Y. H. & Diamond, B. IL-6 contributes to an immune tolerance checkpoint in post germinal center B cells. J. Autoimmun. 38, 1–9 (2012).
Hillion, S., Dueymes, M., Youinou, P. & Jamin, C. IL-6 contributes to the expression of RAGs in human mature B cells. J. Immunol. 179, 6790–6798 (2007).
Rosser, E. C. et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat. Med. 20, 1334–1339 (2014).
Bommert, K., Bargou, R. C. & Stuhmer, T. Signalling and survival pathways in multiple myeloma. Eur. J. Cancer 42, 1574–1580 (2006).
Podar, K., Chauhan, D. & Anderson, K. C. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 23, 10–24 (2009).
Voorhees, P. M. et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 161, 357–366 (2013).
Shah, J. J. et al. Siltuximab (CNTO 328) with lenalidomide, bortezomib and dexamethasone in newly-diagnosed, previously untreated multiple myeloma: an open-label phase I trial. Blood Cancer J. 6, e396 (2016).
Kopf, M., Herren, S., Wiles, M. V., Pepys, M. B. & Kosco-Vilbois, M. H. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J. Exp. Med. 188, 1895–1906 (1998).
Wu, Y. et al. IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation. Int. Immunol. 21, 745–756 (2009).
Nagafuchi, H., Suzuki, N., Mizushima, Y. & Sakane, T. Constitutive expression of IL-6 receptors and their role in the excessive B cell function in patients with systemic lupus erythematosus. J. Immunol. 151, 6525–6534 (1993).
Lee, Y. H., Lee, H. S., Choi, S. J., Ji, J. D. & Song, G. G. The association between interleukin-6 polymorphisms and systemic lupus erythematosus: a meta-analysis. Lupus 21, 60–67 (2012).
Rovin, B. H. et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 68, 2174–2183 (2016).
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 62, 542–552 (2010).
Shirota, Y. et al. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 72, 118–128 (2013).
Narazaki, M., Tanaka, T. & Kishimoto, T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev. Clin. Immunol. 13, 535–551 (2017).
Kang, S., Tanaka, T. & Kishimoto, T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int. Immunol. 27, 21–29 (2015).
Berti, A. et al. Tocilizumab in patients with multisystem Erdheim-Chester disease. Oncoimmunology 6, e1318237 (2017).
Tanaka, T., Narazaki, M. & Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 8, 959–970 (2016).
Kampan, N. C. et al. Immunotherapeutic Interleukin-6 or Interleukin-6 receptor blockade in cancer: challenges and opportunities. Curr. Med. Chem. https://doi.org/10.2174/0929867324666170712160621 (2017).
Acknowledgements
T.K. was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (TRR128, SFB1054-B07 and SyNergy), by the European Research Council (ERC) (EXODUS, CoG 647215) and the German Ministry of Education and Research (T-B interaction in neuromyelitis optica). C.G. was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (SFB877-A10 and Cluster of Excellence 'Inflammation at Interfaces'), and the German Ministry of Education and Research (grant “InTraSig”, project B). S.R.-J. was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (SFB841-C01, SFB877-A1 and Cluster of Excellence 'Inflammation at Interfaces'), and the German Ministry of Education and Research (grant “InTraSig”, project B).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
S.R.-J. has acted as a consultant and speaker for AbbVie, Chugai, Genentech Roche, Pfizer and Sanofi. He also declares that he is an inventor on patents owned by CONARIS Research Institute, which develops the sgp130Fc protein olamkicept together with the company I-Mab. S.R.-J. has stock ownership in CONARIS. All other authors declare no competing interests.
Related links
DATABASES
Glossary
- Multiple myeloma
-
A malignancy of terminally differentiated B lymphocytes associated with elevated immunoglobulin levels secreted by malignant plasma cell clones.
- Castleman disease
-
A spectrum of heterogeneous lymphoproliferative disorders characterized by common lymph node histopathological features (including abnormal germinal centre architecture with prominent follicular dendritic cells) with interleukin-6 (IL-6) overproduction considered as a key mechanism,particularly for the entity multicentric Castleman disease.
- Giant cell arteritis
-
A systemic vasculitis of large and medium vessels with non-necrotizing granulomatous changes (activated macrophages forming multinucleated giant cells and T helper cell infiltration) that occur most frequently in the aorta and the extracranial branches of the external carotid arteries. It is a polygenic and multifactorial disease, and it is characterized by substantially elevated interleukin-6 (IL-6) serum levels.
- Neuromyelitis optica
-
(NMO). An autoimmune disease of the central nervous system distinct from multiple sclerosis. In this disease, astrocytes, which express the water channel protein aquaporin 4 (AQP4), are targeted by an autoantibody to this protein.
- Erdheim–Chester disease
-
Also known as non-Langerhans' cell histiocytosis. A rare, inflammatory myeloid neoplasia characterized by increased production of spumous histiocytes that infiltrate multiple tissues and organs (including bones, heart, lungs and brain) that are frequently surrounded by fibrosis.
- Secondary demyelination
-
The destruction of astrocytes through anti-aquaporin 4 (AQP4) antibodies leads to secondary affection of oligodendrocytes through not entirely understood mechanisms. Oligodendrocytes produce the myelin sheath that surrounds axons in the central nervous system.
- Invariant natural killer T cells
-
(iNKT cells). Innate-like T lymphocytes expressing natural killer (NK) cell surface antigens and an invariant T cell receptor recognizing glycolipid antigens that, upon activation, secrete a variety of cytokines modulating dendritic cells, macrophages, neutrophils and NK cells as well as B and T lymphocytes, therefore orchestrating immune responses in autoimmunity, tumour surveillance and infections.
- γδ T cells
-
Functionally distinct subsets of unconventional T lymphocytes with an invariant γδ T cell receptor that are localized in the liver or at epithelial (digestive tract, respiratory tract and reproductive tract) barriers and possess features of both innate and adaptive immune cells as they secrete various inflammatory cytokines (interferon-γ and interleukin-17) upon sensing of alarm signals (for example, via Toll-like receptors).
- Lymphocytic choriomeningitis virus (LCMV) infection
-
A well-studied rodent viral infectious model that uses different strains to elicit acute or chronic disease. The LCMV model is used to analyse vigorous cytotoxic T cell effector functions, the interplay with other immune cells to eradicate the virus and the generation of immunological memory or T cell exhaustion.
- ACR20
-
The American College of Rheumatology (ACR) clinical score used in patients with rheumatoid arthritis; the score includes physician and patient assessment items. An improvement of the score by 20% is referred to as ACR20; an improvement of the score by 50% is ACR50.
- Nanobody
-
Also known as single-domain antibody. An antibody fragment consisting of a single monomeric variable domain (VH) naturally occurring in the Camelidae family or synthetically derived from the heavy chain of an antibody, combining high antigen affinity in the absence of complement-dependent or cell-mediated cytotoxicity due to the lack of a constant (Fc) region.
Rights and permissions
About this article
Cite this article
Garbers, C., Heink, S., Korn, T. et al. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov 17, 395–412 (2018). https://doi.org/10.1038/nrd.2018.45
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2018.45
This article is cited by
-
Targeting interleukin-6 as a treatment approach for peritoneal carcinomatosis
Journal of Translational Medicine (2024)
-
Genetic evidence for causal effects of immune dysfunction in psychiatric disorders: where are we?
Translational Psychiatry (2024)
-
Translating B cell immunology to the treatment of antibody-mediated allograft rejection
Nature Reviews Nephrology (2024)
-
IL-8 activates fibroblasts to promote the invasion of HNSCC cells via STAT3-MMP1
Cell Death Discovery (2024)
-
Minimizing higher-order aggregation maximizes iron mobilization by small molecules
Nature Chemical Biology (2024)