Key Points
-
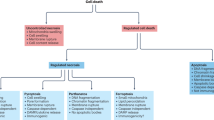
Recent research has identified a series of previously unrecognized regulated cell death modalities beyond apoptosis, including necroptosis, parthanatos, ferroptosis and oxytosis.
-
Several genetic approaches in model systems have implicated these forms of regulated cell death in diverse pathologically relevant conditions.
-
Targeted and phenotypic screening approaches have led to the identification of small molecules that can modulate these pathways.
-
Some of these regulated forms of cell death appear to be intertwined with the innate immune response, thus possibly affecting treatment outcomes with cell-death inducers or inhibitors.
-
Strategies aiming to interfere with multiple cell death pathways might represent the optimal treatment paradigm for translational settings.
-
Owing to their recent discovery, small-molecule modulators of regulated cell death are still in their infancy and therefore require further chemical optimization to reach clinical testing.
Abstract
The discovery of regulated cell death presents tantalizing possibilities for gaining control over the life–death decisions made by cells in disease. Although apoptosis has been the focus of drug discovery for many years, recent research has identified regulatory mechanisms and signalling pathways for previously unrecognized, regulated necrotic cell death routines. Distinct critical nodes have been characterized for some of these alternative cell death routines, whereas other cell death routines are just beginning to be unravelled. In this Review, we describe forms of regulated necrotic cell death, including necroptosis, the emerging cell death modality of ferroptosis (and the related oxytosis) and the less well comprehended parthanatos and cyclophilin D-mediated necrosis. We focus on small molecules, proteins and pathways that can induce and inhibit these non-apoptotic forms of cell death, and discuss strategies for translating this understanding into new therapeutics for certain disease contexts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Laster, S. M., Wood, J. G. & Gooding, L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 (1988).
Vanden Berghe, T., Linkermann, A., Jouan-Lanhouet, S., Walczak, H. & Vandenabeele, P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 135–147 (2014).
Green, D. R. & Levine, B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell 157, 65–75 (2014).
Galluzzi, L., Pietrocola, F., Levine, B. & Kroemer, G. Metabolic control of autophagy. Cell 159, 1263–1276 (2014).
Brinkmann, V. & Zychlinsky, A. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198, 773–783 (2012).
Lupfer, C., Malik, A. & Kanneganti, T. D. Inflammasome control of viral infection. Curr. Opin. Virol. 12, 38–46 (2015).
Mohammad, R. M. et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 35, S78–S103 (2015).
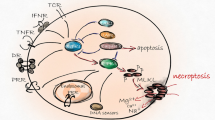
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 (2010).
Sun, L. & Wang, X. A new kind of cell suicide: mechanisms and functions of programmed necrosis. Trends Biochem. Sci. 39, 587–593 (2014).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 (2008). References 10 and 11 describe the first series of small molecules able to prevent necrotic TNF-induced cell death via RIPK1 inhibition.
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Kim, S. K. et al. Upregulated RIP3 expression potentiates MLKL phosphorylation-mediated programmed necrosis in toxic epidermal necrolysis. J. Invest. Dermatol. 135, 2021–2030 (2015).
Ofengeim, D. et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 10, 1836–1849 (2015).
Gunther, C. et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 (2011).
Linkermann, A., Stockwell, B. R., Krautwald, S. & Anders, H. J. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat. Rev. Immunol. 14, 759–767 (2014).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Linkermann, A. et al. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-α-induced shock. Mol. Med. 18, 577–586 (2012).
Newton, K. et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357–1360 (2014).
Jouan-Lanhouet, S. et al. Necroptosis, in vivo detection in experimental disease models. Semin. Cell Dev. Biol. 35, 2–13 (2014).
Dondelinger, Y. et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol. Cell 60, 63–76 (2015).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
Dondelinger, Y. et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 20, 1381–1392 (2013).
Legarda-Addison, D., Hase, H., O'Donnell, M. A. & Ting, A. T. NEMO/IKKγ regulates an early NF-κB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 16, 1279–1288 (2009).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Cai, Z. et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16, 55–65 (2014).
Chen, X. et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 24, 105–121 (2014).
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 (2014).
Wang, H. et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 (2014).
Tenev, T. et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 (2011).
Feoktistova, M. et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011).
Mandal, P. et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 56, 481–495 (2014). This paper demonstrates how small-molecule drugs targeting RIPK3 kinase activity indeed block necroptosis induction, but in some cases induce RIPK3-platform-mediated apoptosis involving caspase 8 and RIPK1 kinase activity.
Hildebrand, J. M. et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl Acad. Sci. USA 111, 15072–15077 (2014).
Teng, X. et al. Structure–activity relationship study of novel necroptosis inhibitors. Bioorg. Med. Chem. Lett. 15, 5039–5044 (2005).
Vandenabeele, P., Grootjans, S., Callewaert, N. & Takahashi, N. Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models. Cell Death Differ. 20, 185–187 (2013).
Takahashi, N. et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 3, e437 (2012).
Degterev, A., Maki, J. L. & Yuan, J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 20, 366 (2013).
Prendergast, G. C., Chang, M. Y., Mandik-Nayak, L., Metz, R. & Muller, A. J. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr. Med. Chem. 18, 2257–2262 (2011).
Xie, T. et al. Structural basis of RIP1 inhibition by necrostatins. Structure 21, 493–499 (2013).
Harris, P. A. et al. Discovery of small molecule RIP1 kinase inhibitors for the treatment of pathologies associated with necroptosis. ACS Med. Chem. Lett. 4, 1238–1243 (2013).
Najjar, M. et al. Structure guided design of potent and selective ponatinib-based hybrid inhibitors for RIPK1. Cell Rep. 10, 1850–1860 (2015).
Fauster, A. et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 6, e1767 (2015).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004).
Wang, Q. et al. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ. Res. 116, 600–611 (2015).
Kaiser, W. J. et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 288, 31268–31279 (2013).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 (2012).
Vitner, E. B. et al. RIPK3 as a potential therapeutic target for Gaucher's disease. Nat. Med. 20, 204–208 (2014).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). In this paper, a pharmacoproteomics approach based on a necroptosis-inhibiting drug (NSA) led to the successful identification of the execution protein MLKL, which is regulated by RIPK3-dependent phosphorylation.
Li, J. et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012).
Cauwels, A., Janssen, B., Waeytens, A., Cuvelier, C. & Brouckaert, P. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat. Immunol. 4, 387–393 (2003).
Duprez, L. et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 (2011).
Wu, J. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006 (2013).
Polykratis, A. et al. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014).
Yang, W. S. & Stockwell, B. R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245 (2008).
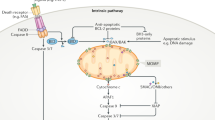
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012). In this paper, the term ferroptosis is coined, and it is shown that a functional system X c− is required to maintain glutathione levels to inhibit this form of cell death in a subset of cancer cell lines.
Sato, H., Tamba, M., Ishii, T. & Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 274, 11455–11458 (1999).
Conrad, M. & Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system Xc−: cystine supplier and beyond. Amino Acids 42, 231–246 (2012).
Ursini, F., Maiorino, M., Valente, M., Ferri, L. & Gregolin, C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 710, 197–211 (1982).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014). This paper shows that GPX4 is the limiting glutathione-utilizing enzyme required for the prevention of ferroptosis in cancer cells.
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014). This paper shows that the ferroptosis inhibitor liproxstatin-1 protects mice from acute renal failure induced by inducible Gpx4 ablation as well as from hepatic IRI.
Linkermann, A. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl Acad. Sci. USA 111, 16836–16841 (2014). This paper indicates that in vivo inhibition of ferroptosis leads to a protective effect in a pathophysiological setting, such as IRI in the kidney.
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell. Metab. 8, 237–248 (2008). This paper describes the first conditional-knockout model of the ferroptosis regulator GPX4 in cells and mice, which were further used for the development of liproxstatin-1 and for assessing the importance of ferroptosis in different tissues.
Yoo, S. E. et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 52, 1820–1827 (2012).
Chen, L., Na, R., Gu, M., Richardson, A. & Ran, Q. Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer's disease. J. Neurochem. 107, 197–207 (2008).
Yoo, M. H. et al. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer's disease. Antioxid. Redox Signal 12, 819–827 (2010).
Ufer, C. et al. Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development. Genes Dev. 22, 1838–1850 (2008).
Hauser, D. N., Dukes, A. A., Mortimer, A. D. & Hastings, T. G. Dopamine quinone modifies and decreases the abundance of the mitochondrial selenoprotein glutathione peroxidase 4. Free Radic. Biol. Med. 65, 419–427 (2013).
Bellinger, F. P. et al. Changes in selenoprotein P in substantia nigra and putamen in Parkinson's disease. J. Parkinsons Dis. 2, 115–126 (2012).
Bellinger, F. P. et al. Glutathione peroxidase 4 is associated with neuromelanin in substantia nigra and dystrophic axons in putamen of Parkinson's brain. Mol. Neurodegener. 6, 8 (2011).
Skouta, R. et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 136, 4551–4556 (2014). Ferrostatins with improved properties are reported in this paper to be effective in several cellular disease models. These ferrostatins block lipid peroxidation, but not by inhibiting mitochondrial ROS production or preventing lysosomal membrane permeabilization.
Wirth, E. K. et al. Cerebellar hypoplasia in mice lacking selenoprotein biosynthesis in neurons. Biol. Trace Elem. Res. 158, 203–210 (2014).
Roth, T. L. et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228 (2014).
Korade, Z. et al. Antioxidant supplementation ameliorates molecular deficits in Smith–Lemli–Opitz syndrome. Biol. Psychiatry 75, 215–222 (2014).
Ueta, T. et al. Glutathione peroxidase 4 is required for maturation of photoreceptor cells. J. Biol. Chem. 287, 7675–7682 (2012).
Sengupta, A. et al. Targeted disruption of glutathione peroxidase 4 in mouse skin epithelial cells impairs postnatal hair follicle morphogenesis that is partially rescued through inhibition of COX-2. J. Invest. Dermatol. 133, 1731–1741 (2013).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Wortmann, M. et al. Combined deficiency in glutathione peroxidase 4 and vitamin E causes multiorgan thrombus formation and early death in mice. Circ. Res. 113, 408–417 (2013).
Woo, J. H. et al. Elucidating compound mechanism of action by network perturbation analysis. Cell 162, 441–451 (2015).
Bannai, S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J. Biol. Chem. 261, 2256–2263 (1986).
Mandal, P. K. et al. System Xc− and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J. Biol. Chem. 285, 22244–22253 (2010).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015). This paper implicates ferroptosis as a non-canonical tumour suppressive function of p53 via p53-mediated transcriptional inhibition of system X c−.
Hayano, M., Yang, W. S., Corn, C. K., Pagano, N. C. & Stockwell, B. R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. http://dx.doi.org/10.1038/cdd.2015.93 (2015).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 (2003).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015). This paper suggests that inhibiting glutaminolysis might be a putative anti-ferroptotic pharmacological approach and that cells that preferably use glutaminolysis for energy production are more sensitive to ferroptosis.
Tretter, L. & Adam-Vizi, V. Generation of reactive oxygen species in the reaction catalyzed by α-ketoglutarate dehydrogenase. J. Neurosci. 24, 7771–7778 (2004).
Bae, Y. S. et al. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275, 10527–10531 (2000).
Han, C. Y. et al. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J. Biol. Chem. 287, 10379–10393 (2012).
Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513, 251–255 (2014).
Dixon, S. J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Dixon, S. J. et al. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3, e02523 (2014). Here, inhibition of system X c− by erastin or sorafenib is shown to be associated with endoplasmic reticulum stress and glutathione- specific γ-glutamylcyclotransferase 1 upregulation involved in glutathione degradation.
Yagoda, N. et al. RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447, 864–868 (2007).
Louandre, C. et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer 133, 1732–1742 (2013).
Chung, W. J. et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 25, 7101–7110 (2005).
Chen, R. S. et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/β-catenin pathway. Oncogene 28, 599–609 (2009).
Nagano, O., Okazaki, S. & Saya, H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 32, 5191–5198 (2013).
Timmerman, L. A. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013).
Keldsen, N., Havsteen, H., Vergote, I., Bertelsen, K. & Jakobsen, A. Altretamine (hexamethylmelamine) in the treatment of platinum-resistant ovarian cancer: a phase II study. Gynecol. Oncol. 88, 118–122 (2003).
Lee, Y. et al. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat. Neurosci. 16, 1392–1400 (2013).
Virag, L., Robaszkiewicz, A., Rodriguez-Vargas, J. M. & Oliver, F. J. Poly(ADP-ribose) signaling in cell death. Mol. Aspects Med. 34, 1153–1167 (2013).
Curtin, N. J. & Szabo, C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol. Aspects Med. 34, 1217–1256 (2013).
Mashimo, M., Kato, J. & Moss, J. ADP-ribosyl-acceptor hydrolase 3 regulates poly (ADP-ribose) degradation and cell death during oxidative stress. Proc. Natl Acad. Sci. USA 110, 18964–18969 (2013).
Andrabi, S. A., Dawson, T. M. & Dawson, V. L. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann. NY Acad. Sci. 1147, 233–241 (2008).
Wang, Y. et al. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci. Signal. 4, ra20 (2011).
Andrabi, S. A. et al. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nat. Med. 17, 692–699 (2011). In this paper, the first endogenous inhibitor of parthanatos is described, indicating that interfering with PAR polymer signalling is a putative treatment for neurodegenerative disease.
Graziani, G. & Szabo, C. Clinical perspectives of PARP inhibitors. Pharmacol. Res. 52, 109–118 (2005).
del Moral, R. M. et al. PARP inhibition attenuates histopathological lesion in ischemia/reperfusion renal mouse model after cold prolonged ischemia. ScientificWorldJournal 2013, 486574 (2013).
Sahaboglu, A. et al. PARP1 gene knock-out increases resistance to retinal degeneration without affecting retinal function. PLoS ONE 5, e15495 (2010).
Jagtap, P. et al. Novel phenanthridinone inhibitors of poly (adenosine 5′-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents. Crit. Care Med. 30, 1071–1082 (2002).
Jagtap, P. G. et al. The discovery and synthesis of novel adenosine substituted 2,3-dihydro-1H-isoindol-1-ones: potent inhibitors of poly(ADP-ribose) polymerase-1 (PARP-1). Bioorg. Med. Chem. Lett. 14, 81–85 (2004).
Jagtap, P. G. et al. Discovery of potent poly(ADP-ribose) polymerase-1 inhibitors from the modification of indeno[1,2-c]isoquinolinone. J. Med. Chem. 48, 5100–5103 (2005).
Morrow, D. A. et al. A randomized, placebo-controlled trial to evaluate the tolerability, safety, pharmacokinetics, and pharmacodynamics of a potent inhibitor of poly(ADP-ribose) polymerase (INO-1001) in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of the TIMI 37 trial. J. Thromb. Thrombolysis 27, 359–364 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Andrabi, S. A. et al. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl Acad. Sci. USA 111, 10209–10214 (2014). Although parthanatos still lacks a clear core cell death pathway, this paper indicates that overactivation of PARP1 is caused by PAR-dependent inhibition of glycolysis through the inhibition of HK, explaining the bioenergetic collapse that results in necrotic cell death.
Devalaraja-Narashimha, K., Diener, A. M. & Padanilam, B. J. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am. J. Physiol. Renal Physiol. 297, F749–F759 (2009).
Millay, D. P. et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 14, 442–447 (2008).
Jobe, S. M. et al. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood 111, 1257–1265 (2008).
Fujimoto, K., Chen, Y., Polonsky, K. S. & Dorn, G. W. 2nd. Targeting cyclophilin D and the mitochondrial permeability transition enhances β-cell survival and prevents diabetes in Pdx1 deficiency. Proc. Natl Acad. Sci. USA 107, 10214–10219 (2010).
Forte, M. et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl Acad. Sci. USA 104, 7558–7563 (2007).
Javadov, S. & Kuznetsov, A. Mitochondrial permeability transition and cell death: the role of cyclophilin D. Front. Physiol. 4, 76 (2013).
Giorgio, V. et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl Acad. Sci. USA 110, 5887–5892 (2013).
Bonora, M., Bravo- San Pedro, J. M., Kroemer, G., Galluzzi, L. & Pinton, P. Novel insights into the mitochondrial permeability transition. Cell Cycle 13, 2666–2670 (2014).
Green, D. R., Galluzzi, L. & Kroemer, G. Cell biology. Metabolic control of cell death. Science 345, 1250256 (2014).
Vaseva, A. V. et al. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548 (2012).
Karch, J. & Molkentin, J. D. Is p53 the long-sought molecular trigger for cyclophilin D-regulated mitochondrial permeability transition pore formation and necrosis? Circ. Res. 111, 1258–1260 (2012).
Nakagawa, T. et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 (2005).
Baines, C. P. et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 (2005).
Schinzel, A. C. et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl Acad. Sci. USA 102, 12005–12010 (2005). References 126–128 characterize the requirement of CypD for the formation of the MPTP, and its inhibition as a pharmacologically amenable approach.
Zhang, L. H., Youn, H. D. & Liu, J. O. Inhibition of cell cycle progression by the novel cyclophilin ligand sanglifehrin A is mediated through the NFκB-dependent activation of p53. J. Biol. Chem. 276, 43534–43540 (2001).
Clarke, S. J., McStay, G. P. & Halestrap, A. P. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J. Biol. Chem. 277, 34793–34799 (2002).
Linkermann, A. et al. Two independent pathways of regulated necrosis mediate ischemia–reperfusion injury. Proc. Natl Acad. Sci. USA 110, 12024–12029 (2013).
Nighoghossian, N. et al. Cyclosporine in acute ischemic stroke. Neurology 84, 2216–2223 (2015).
Keogh, A. Calcineurin inhibitors in heart transplantation. J. Heart Lung Transplant 23, S202–S206 (2004).
Zhao, H. et al. Necroptosis and parthanatos are involved in remote lung injury after receiving ischemic renal allografts in rats. Kidney Int. 87, 738–748 (2015).
Takahashi, N. et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 513, 95–99 (2014).
Dannappel, M. et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513, 90–94 (2014).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Ch'en, I. L., Tsau, J. S., Molkentin, J. D., Komatsu, M. & Hedrick, S. M. Mechanisms of necroptosis in T cells. J. Exp. Med. 208, 633–641 (2011).
Moriwaki, K. & Chan, F. K. Necrosis-dependent and independent signaling of the RIP kinases in inflammation. Cytokine Growth Factor Rev. 25, 167–174 (2014).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010).
Kang, T. B., Yang, S. H., Toth, B., Kovalenko, A. & Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 (2013). This study puts forward for the first time the possible and complex interaction between the necrosome and inflammasome machinery.
Moriwaki, K., Bertin, J., Gough, P. J. & Chan, F. K. A. RIPK3-caspase 8 complex mediates atypical pro-IL-1β processing. J. Immunol. 194, 1938–1944 (2015).
Lawlor, K. E. et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 6, 6282 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. 526, 666–671 Nature (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. 526, 660–665 Nature (2015).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Tan, S., Schubert, D. & Maher, P. Oxytosis: a novel form of programmed cell death. Curr. Top. Med. Chem. 1, 497–506 (2001).
Li, Y., Maher, P. & Schubert, D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 19, 453–463 (1997).
Pallast, S. et al. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J. Cereb. Blood Flow Metab. 30, 1157–1167 (2010).
Pallast, S., Arai, K., Wang, X., Lo, E. H. & van Leyen, K. 12/15-lipoxygenase targets neuronal mitochondria under oxidative stress. J. Neurochem. 111, 882–889 (2009).
Yigitkanli, K. et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann. Neurol. 73, 129–135 (2013).
van Leyen, K. et al. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37, 3014–3018 (2006). This study demonstrates that glutathione depletion leads to lipid peroxidation and cell death in neuronal cells — a phenomenon later termed as oxytosis, which is related to ferroptosis.
Jin, G. et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke 39, 2538–2543 (2008).
Oxler, E. M., Dolga, A. & Culmsee, C. AIF depletion provides neuroprotection through a preconditioning effect. Apoptosis 17, 1027–1038 (2012).
Mahoney, D. J. et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl Acad. Sci. USA 105, 11778–11783 (2008).
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Varfolomeev, E. et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 (2007).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007).
Petersen, S. L. et al. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12, 445–456 (2007).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Lin, Y., Devin, A., Rodriguez, Y. & Liu, Z. G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 (1999).
Feng, S. et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 19, 2056–2067 (2007).
O'Donnell, M. A. et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 13, 1437–1442 (2011).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Zhao, J. et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl Acad. Sci. USA 109, 5322–5327 (2012).
Fulda, S. Smac mimetics as IAP antagonists. Semin. Cell Dev. Biol. 39, 132–138 (2015).
Kobayashi, S. et al. Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function. J. Biol. Chem. 290, 8778–8788 (2015).
Bannai, S. & Kitamura, E. Transport interaction of l-cystine and l-glutamate in human diploid fibroblasts in culture. J. Biol. Chem. 255, 2372–2376 (1980).
Gout, P. W., Buckley, A. R., Simms, C. R. & Bruchovsky, N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the Xc− cystine transporter: a new action for an old drug. Leukemia 15, 1633–1640 (2001).
Patel, S. A., Warren, B. A., Rhoderick, J. F. & Bridges, R. J. Differentiation of substrate and non-substrate inhibitors of transport system Xc−: an obligate exchanger of l -glutamate and l-cystine. Neuropharmacology 46, 273–284 (2004).
Fatokun, A. A., Dawson, V. L. & Dawson, T. M. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br. J. Pharmacol. 171, 2000–2016 (2014).
Shall, S. Proceedings: experimental manipulation of the specific activity of poly(ADP-ribose) polymerase. J. Biochem 77, 2p (1975).
Fatokun, A. A., Liu, J. O., Dawson, V. L. & Dawson, T. M. Identification through high-throughput screening of 4′-methoxyflavone and 3′,4′-dimethoxyflavone as novel neuroprotective inhibitors of parthanatos. Br. J. Pharmacol. 169, 1263–1278 (2013).
Greco, R. et al. Neuroprotection by the PARP inhibitor PJ34 modulates cerebral and circulating RAGE levels in rats exposed to focal brain ischemia. Eur. J. Pharmacol. 744, 91–97 (2014).
Wang, J. et al. Inhibition of poly (ADP-ribose) polymerase and inducible nitric oxide synthase protects against ischemic myocardial damage by reduction of apoptosis. Mol. Med. Rep. 11, 1768–1776 (2015).
Ha, H. C. & Snyder, S. H. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl Acad. Sci. USA 96, 13978–13982 (1999).
Meier, H. L., Ballough, G. P., Forster, J. S. & Filbert, M. G. Benzamide, a poly(ADP-ribose) polymerase inhibitor, is neuroprotective against soman-induced seizure-related brain damage. Ann. NY Acad. Sci. 890, 330–335 (1999).
Purnell, M. R. & Whish, W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem. J. 185, 775–777 (1980).
Szabo, G. et al. INO-1001 a novel poly(ADP-ribose) polymerase (PARP) inhibitor improves cardiac and pulmonary function after crystalloid cardioplegia and extracorporal circulation. Shock 21, 426–432 (2004).
d'Avila, J. C. et al. Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J. Neuroinflamm. 9, 31 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2006).
US National Library of Medicine. ClinicalTrials.gov [online], (2005).
van Leyen, K. et al. Novel lipoxygenase inhibitors as neuroprotective reagents. J. Neurosci. Res. 86, 904–909 (2008).
Niki, E. & Traber, M. G. A history of vitamin E. Ann. Nutr. Metab. 61, 207–212 (2012).
Griffith, O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J. Biol. Chem. 257, 13704–13712 (1982).
Harris, I. S. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222 (2015).
Kim, S. Y. et al. Inhibition of cyclophilin D by cyclosporin A promotes retinal ganglion cell survival by preventing mitochondrial alteration in ischemic injury. Cell Death Dis. 5, e1105 (2014).
Teixeira, G. et al. Synergistic protective effect of cyclosporin A and rotenone against hypoxia–reoxygenation in cardiomyocytes. J. Mol. Cell Cardiol. 56, 55–62 (2013).
Wang, X. et al. Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury. J. Neurosci. 29, 2588–2596 (2009).
Hausenloy, D., Wynne, A., Duchen, M. & Yellon, D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 109, 1714–1717 (2004).
Strom, E. et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat. Chem. Biol. 2, 474–479 (2006).
Acknowledgements
The authors are grateful to B. Proneth (Helmholtz Zentrum München) for help in the preparation of figures and to M. Lamkanfi (Flanders Institute for Biotechnology, Ghent University) for critically reading and comments on the inflammasome section of this manuscript. M.C. is partially supported by the Deutsche Forschungsgemeinschaft (DFG) research grant CO 291/2-3 and DFG Priority Programme 1710 (CO 291/5-1), the Human Frontier Science Program (HFSP) RGP0013/14, and the m4 Award (Bavarian Ministry of Economic Affairs). B.R.S. is an Early Career Scientist of the Howard Hughes Medical Institute and is supported by New York State Stem Cell Science (NYSTEM) contract No. C026715, and the US National Institutes of Health (NIH) (grants 5R01CA097061, 1R21CA177591 and R01CA161061). The authors thank J. Decatur and the Columbia Chemistry NMR core facility (US National Science Foundation grant CHE 0840451 and NIH grant 1S10RR025431-01A1). Research in the P.V. unit is supported by Belgian grants (Interuniversity Attraction Poles, IAP 7/32), Flemish grants (Research Foundation Flanders, FWO G.0875.11, FWO G.0973.11N, FWO G.0A45.12 N, FWO G.0172.12, FWO G.0787.13N, G0C3114N, FWO KAN 31528711, and Foundation against Cancer 2012–188), Ghent University grants (MRP, GROUP-ID consortium) and grants from the Flanders Institute for Biotechnology. P.V. holds a Methusalem grant (BOF09/01M00709) from the Flemish Government.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
M.C. and B.R.S. have filed patent applications for some of the molecules described in the article.
Glossary
- Pseudokinase
-
A protein that contains a catalytically inactive kinase domain. The loss of activity is attributed to the lack of at least one of three motifs (namely, VAIK, HRD or DFG) that are normally required for catalysis.
- Fluorescence polarization assay
-
An assay used to analyse macromolecular interactions: one of the studied molecules is labelled with a fluorophore, which allows the measurement of the ratio of bound to unbound molecule, thus directly providing an estimation of molecular affinity.
- Type II kinase inhibitor
-
An inhibitor that binds competitively with ATP, using the Asp-Phe-Gly (DFG)-out conformation.
- DFG-out conformation
-
A state in which the kinase adopts a catalytically inactive conformation whereby the Asp-Phe-Gly (DFG) motif at the amino terminus of the activation loop faces outwards.
- Gaucher disease
-
A genetic disorder characterized by deficiency of the enzyme glucocerebrosidase, leading to the accumulation of sphingolipids in certain organs. The disease is also characterized by enlargement of the liver and the spleen, low blood cell count and anaemia.
- Glutaminolysis
-
A series of metabolic reactions based on the use of glutamine to produce energy and substrates to replenish the tricarboxylic acid cycle.
- Pentose phosphate pathway
-
(PPP). A series of metabolic reactions converting glucose into precursors for nucleotide biosynthesis and reducing equivalents in the form of NADPH/H+.
- Network perturbation analysis
-
A hybrid computational and experimental approach for mode-of-action analysis. Compound-induced dysregulations in the expression of genes in a regulatory network are scored, allowing compounds with a similar mode of action to be clustered.
- Peroxide tone
-
The steady-state level of peroxides or lipid peroxides in a given cell.
- Harlequin neuronal cells
-
Neuronal cells derived from the Harlequin mouse. These animals contain a proviral insertion in the apoptosis inducing factor (Aif) gene, leading to approximately 70% reduction of AIF expression.
Rights and permissions
About this article
Cite this article
Conrad, M., Angeli, J., Vandenabeele, P. et al. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 15, 348–366 (2016). https://doi.org/10.1038/nrd.2015.6
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2015.6
This article is cited by
-
Targeting immunogenic cell stress and death for cancer therapy
Nature Reviews Drug Discovery (2024)
-
Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity
Nature Reviews Cancer (2024)
-
Natural inhibitor found for cell death by ferroptosis
Nature (2024)
-
Circ-STC2 promotes the ferroptosis of nucleus pulposus cells via targeting miR-486-3p/TFR2 axis
Journal of Orthopaedic Surgery and Research (2023)
-
SPINK4 promotes colorectal cancer cell proliferation and inhibits ferroptosis
BMC Gastroenterology (2023)