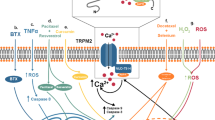
Key Points
-
Nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) are labile gaseous mediators that have multiple biological functions in tumour cells and in the host tissue. Each of these gases is produced by specific enzyme systems and regulates (among other aspects) cell viability, cell division, mitochondrial activity, angiogenesis and vascular tone.
-
Upregulation of the various gasotransmitter-producing enzymes occurs in many tumours. Most commonly, NO is overproduced by upregulation of inducible NO synthase (iNOS); CO is overproduced by haem oxygenase 1 (HO1); and H2S is overproduced by cystathionine-β-synthase (CBS).
-
Selective genetic silencing or pharmacological inhibition of iNOS, HO1 or CBS has been shown to exert anticancer effects in various in vitro and in vivo models. Many of these approaches also sensitize the tumour to chemotherapy and/or radiotherapy.
-
Because of the bell-shaped pharmacological character of the gasotransmitters, not only inhibition of gasotransmitter biosynthesis, but also elevation of gasotransmitter levels beyond a certain threshold can exert antitumour effects; preclinical data show that tumour-targeted NO donors, CO donors or CO inhalation therapy, and H2S donors of various classes exert antitumour effects.
-
Although the clinical translation of the findings of gasotransmitters in the field of tumour biology has been slow, several compounds can be identified that may be suitable for clinical repurposing and translational research activity.
Abstract
The three endogenous gaseous transmitters — nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) — regulate a number of key biological functions. Emerging data have revealed several new mechanisms for each of these three gasotransmitters in tumour biology. It is now appreciated that they show bimodal pharmacological character in cancer, in that not only the inhibition of their biosynthesis but also elevation of their concentration beyond a certain threshold can exert anticancer effects. This Review discusses the role of each gasotransmitter in cancer and the effects of pharmacological agents — some of which are in early-stage clinical studies — that modulate the levels of each gasotransmitter. A clearer understanding of the pharmacological character of these three gases and the mechanisms underlying their biological effects is expected to guide further clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 6, 3051–3064 (1992).
Stuehr, D. J. Mammalian nitric oxide synthases. Biochim. Biophys. Acta 1411, 217–230 (1999).
Liaudet, L., Soriano, F. G. & Szabo, C. Biology of nitric oxide signaling. Crit. Care Med. 28, N37–52 (2000).
Ignarro, L. J. Nitric Oxide: Biology and Pathobiology 2nd edn (Academic Press, 2009).
Southan, G. J. & Szabo, C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 51, 383–394 (1996).
Joubert, J. & Malan, S. F. Novel nitric oxide synthase inhibitors: a patent review. Expert Opin. Ther. Pat. 21, 537–560 (2011).
Szabo, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6, 662–680 (2007).
Pacher, P., Beckman, J. S. & Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 (2007).
Kröncke, K. D., Fehsel, K. & Kolb-Bachofen, V. Nitric oxide: cytotoxicity versus cytoprotection — how, why, when, and where? Nitric Oxide 1, 107–120 (1997).
Wink, D. A. et al. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 3, 203–213 (2001).
Thomas, D. D. et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 45, 18–31 (2008).
Calabrese, V. et al. Nitric oxide in cell survival: a janus molecule. Antioxid. Redox Signal. 11, 2717–2739 (2009).
Hibbs, J. B. Jr, Taintor, R. R. & Vavrin, Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235, 473–476 (1987).
Stuehr, D. J. & Nathan, C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169, 1543–1555 (1989). A pioneering study that implicates NO in immune activation-mediated killing of tumour cells in vitro.
Cui, S., Reichner, J. S., Mateo, R. B. & Albina, J. E. Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 54, 2462–2467 (1994).
MacMicking, J., Xie, Q. W. & Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15, 323–350 (1997).
Farias-Eisner, R., Sherman, M. P., Aeberhard, E. & Chaudhuri, G. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc. Natl Acad. Sci. USA 91, 9407–9411 (1994). Another pioneering study that implicates NO in immune activation-mediated killing of tumour cells, in vivo.
Wang, B. et al. Intact nitric oxide synthase II gene is required for interferon-β-mediated suppression of growth and metastasis of pancreatic adenocarcinoma. Cancer Res. 61, 71–75 (2001).
Wei, D. et al. Direct demonstration of negative regulation of tumor growth and metastasis by host-inducible nitric oxide synthase. Cancer Res. 63, 3855–3859 (2003).
Menon, C. et al. Tumoricidal activity of high-dose tumor necrosis factor-α is mediated by macrophage-derived nitric oxide burst and permanent blood flow shutdown. Int. J. Cancer 123, 464–475 (2008).
Kawakami, K., Kawakami, M. & Puri, R. K. Nitric oxide accelerates interleukin-13 cytotoxin-mediated regression in head and neck cancer animal model. Clin. Cancer Res. 10, 5264–5270 (2004).
Wang, B. et al. A novel model system for studying the double-edged roles of nitric oxide production in pancreatic cancer growth and metastasis. Oncogene 22, 1771–1782 (2003).
Shi, Q. et al. Influence of nitric oxide synthase II gene disruption on tumor growth and metastasis. Cancer Res. 60, 2579–2583 (2000).
Konopka, T. E. et al. Nitric oxide synthase II gene disruption: implications for tumor growth and vascular endothelial growth factor production. Cancer Res. 61, 3182–3187 (2001).
DiNapoli, M. R., Calderon, C. L. & Lopez, D. M. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J. Exp. Med. 183, 1323–1329 (1996).
Weigert, A. & Brüne, B. Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide 19, 95–102 (2008).
Böhle, A. & Brandau, S. Immune mechanisms in bacillus Calmette–Guérin immunotherapy for superficial bladder cancer. J. Urol. 170, 964–969 (2003).
Hosseini, A. et al. Enhanced formation of nitric oxide in bladder carcinoma in situ and in BCG treated bladder cancer. Nitric Oxide 15, 337–343 (2006).
Shah, G. et al. iNOS expression and NO production contribute to the direct effects of BCG on urothelial carcinoma cell biology. Urol. Oncol. 32, 45.e1–45.e9 (2014).
Mills, C. D., Shearer, J., Evans, R. & Caldwell, M. D. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J. Immunol. 149, 2709–2714 (1992).
Ma, Q., Hoper, M., Anderson, N. & Rowlands, B. J. Effect of supplemental L-arginine in a chemical-induced model of colorectal cancer. World J. Surg. 20, 1087–1091 (1996).
Yeh, C. L., Pai, M. H., Li, C. C., Tsai, Y. L. & Yeh, S. L. Effect of arginine on angiogenesis induced by human colon cancer: in vitro and in vivo studies. J. Nutr. Biochem. 21, 538–543 (2010).
Brittenden, J., Heys, S. D., Ross, J., Park, K. G. & Eremin, O. Natural cytotoxicity in breast cancer patients receiving neoadjuvant chemotherapy: effects of L-arginine supplementation. Eur. J. Surg. Oncol. 20, 467–472 (1994).
Heys, S. D. et al. Dietary supplementation with L-arginine: modulation of tumour-infiltrating lymphocytes in patients with colorectal cancer. Br. J. Surg. 84, 238–241 (1997).
Buijs, N. et al. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am. J. Clin. Nutr. 92, 1151–1156 (2010).
Zhao, H. et al. Randomized clinical trial of arginine-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing gastric cancer surgery. J. Cancer Res. Clin. Oncol. 139, 1465–1470 (2013).
Ma, Q., Wang, Y., Gao, X., Ma, Z. & Song, Z. L-arginine reduces cell proliferation and ornithine decarboxylase activity in patients with colorectal adenoma and adenocarcinoma. Clin. Cancer Res. 13, 7407–7412 (2007). This in vivo study shows that L -arginine upregulates NO production in colorectal tumours to confer inhibitory effects on tumour progression.
Rahat, M. A. & Hemmerlein, B. Macrophage–tumor cell interactions regulate the function of nitric oxide. Front. Physiol. 4, 144 (2013).
Huerta, S., Chilka, S. & Bonavida, B. Nitric oxide donors: novel cancer therapeutics. Int. J. Oncol. 33, 909–927 (2008).
Yasuda, H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: nitric oxide donor as a therapeutic enhancer. Nitric Oxide 19, 205–216 (2008).
Jeannin, J. F. et al. Nitric oxide-induced resistance or sensitization to death in tumor cells. Nitric Oxide 19, 158–163 (2008).
De Ridder, M., Verellen, D., Verovski, V. & Storme, G. Hypoxic tumor cell radiosensitization through nitric oxide. Nitric Oxide 19, 164–169 (2008).
Sonveaux, P., Jordan, B. F., Gallez, B. & Feron, O. Nitric oxide delivery to cancer: why and how? Eur. J. Cancer 45, 1352–1369 (2009).
Reynolds, M. M. et al. Applications for nitric oxide in halting proliferation of tumor cells. Biochem. Biophys. Res. Commun. 431, 647–651 (2013).
Rigas, B. & Williams, J. L. NO-donating NSAIDs and cancer: an overview with a note on whether NO is required for their action. Nitric Oxide 19, 199–204 (2008).
Findlay, V. J. et al. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol. Pharmacol. 65, 1070–1079 (2004).
McMurtry, V. et al. JS-K, a nitric oxide-releasing prodrug, induces breast cancer cell death while sparing normal mammary epithelial cells. Int. J. Oncol. 38, 963–971 (2011). This study demonstrates the anticancer efficacy of a tumour-targeted NO donor in vivo.
Duan, S., Cai, S., Yang, Q. & Forrest, M. L. Multi-arm polymeric nanocarrier as a nitric oxide delivery platform for chemotherapy of head and neck squamous cell carcinoma. Biomaterials 33, 3243–3253 (2012).
Carpenter, A. W. & Schoenfisch, M. H. Nitric oxide release: part II. Therapeutic applications. Chem. Soc. Rev. 41, 3742–3752 (2012).
Rapozzi, V. et al. Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide 30, 26–35 (2013).
Heilman, B. & Mascharak, P. K. Light-triggered nitric oxide delivery to malignant sites and infection. Philos. Trans. A Math. Phys. Eng. Sci. 371, 20120368 (2013).
Sharma, K. & Chakrapani, H. Site-directed delivery of nitric oxide to cancers. Nitric Oxide 43, 8–16 (2014). This study demonstrates the anticancer efficacy of tumour-targeted NO donors.
Radomski, M. W., Jenkins, D. C., Holmes, L. & Moncada, S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 51, 6073–6078 (1991).
Thomsen, L. L. & Miles, D. W. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev. 17, 107–118 (1998).
Jahani-Asl, A. & Bonni, A. iNOS: a potential therapeutic target for malignant glioma. Curr. Mol. Med. 13, 1241–1249 (2013).
Heinecke, J. L. et al. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc. Natl Acad. Sci. USA 111, 6323–6328 (2014). This study demonstrates the suppression of tumour growth by selective iNOS inhibition with aminoguanidine in vivo and demonstrates the pro-proliferative, tumour-autonomous roles of iNOS-derived NO.
Granados-Principal, S. et al. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 17, 25 (2015).
Jenkins, D. C. et al. Roles of nitric oxide in tumor growth. Proc. Natl Acad. Sci. USA 92, 4392–4396 (1995). This study pioneered the concept that iNOS in tumour cells can promote tumour growth in vivo , at least in part through stimulation of tumour angiogenesis.
Kostourou, V. et al. The role of tumour-derived iNOS in tumour progression and angiogenesis. Br. J. Cancer 104, 83–90 (2011).
Sikora, A. G. et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin. Cancer Res. 16, 1834–1844 (2010).
Juang, S. H. et al. Use of retroviral vectors encoding murine inducible nitric oxide synthase gene to suppress tumorigenicity and cancer metastasis of murine melanoma. Cancer Biother. Radiopharm. 12, 167–175 (1997).
Papapetropoulos, A., García-Cardeña, G., Madri, J. A. & Sessa, W. C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Invest. 100, 3131–3139 (1997). This study defined the role of NO as a pro-angiogenic mediator.
Sessa, W. C. Molecular control of blood flow and angiogenesis: role of nitric oxide. J. Thromb. Haemost. 7, s35–37 (2009).
Ziche, M. & Morbidelli, L. Molecular regulation of tumour angiogenesis by nitric oxide. Eur. Cytokine Netw. 20, 164–170 (2009).
Thomsen, L. L. et al. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 57, 3300–3304 (1997). This study shows that selective iNOS inhibition with 1400W suppresses in vivo tumour growth.
Camp, E. R. et al. Roles of nitric oxide synthase inhibition and vascular endothelial growth factor receptor-2 inhibition on vascular morphology and function in an in vivo model of pancreatic cancer. Clin. Cancer Res. 12, 2628–2633 (2006).
Lopez-Rivera, E. et al. Inducible nitric oxide synthase drives mTOR pathway activation and proliferation of human melanoma by reversible nitrosylation of TSC2. Cancer Res. 74, 1067–1078 (2014).
Wang, G. Y., Ji, B., Wang, X. & Gu, J. H. Anti-cancer effect of iNOS inhibitor and its correlation with angiogenesis in gastric cancer. World J. Gastroenterol. 11, 3830–3833 (2005).
Tozer, G. M. et al. Nitric oxide synthase inhibition enhances the tumor vascular-damaging effects of combretastatin A-4 3-O-phosphate at clinically relevant doses. Clin. Cancer Res. 15, 3781–3790 (2009).
Cardnell, R. J. & Mikkelsen, R. B. Nitric oxide synthase inhibition enhances the antitumor effect of radiation in the treatment of squamous carcinoma xenografts. PLoS ONE 6, e20147 (2011).
Wood, P. J. et al. Modification of energy metabolism and radiation response of a murine tumour by changes in nitric oxide availability. Biochem. Biophys. Res. Commun. 192, 505–510 (1993). This study provides evidence that modulation of NO homeostasis can affect tumour radiation responses in vivo.
Wood, P. J. et al. Induction of hypoxia in experimental murine tumors by the nitric oxide synthase inhibitor, NG-nitro-L-arginine. Cancer Res. 54, 6458–6463 (1994).
Bolton, W. K. et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am. J. Nephrol. 24, 32–40 (2004).
Brindicci, C., Ito, K., Torre, O., Barnes, P. J. & Kharitonov, S. A. Effects of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on nitric oxide production and its metabolites in healthy control subjects, healthy smokers, and COPD patients. Chest 135, 353–367 (2009).
López, A. et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit. Care Med. 32, 21–30 (2004).
Alderton, W. K. et al. GW274150 and GW273629 are potent and highly selective inhibitors of inducible nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 145, 301–312 (2005).
Singh, D. et al. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am. J. Respir. Crit. Care Med. 176, 988–993 (2007).
Høivik, H. O. et al. Lack of efficacy of the selective iNOS inhibitor GW274150 in prophylaxis of migraine headache. Cephalalgia 30, 1458–1467 (2010).
Seymour, M. et al. Ultrasonographic measures of synovitis in an early phase clinical trial: a double-blind, randomised, placebo and comparator controlled phase IIa trial of GW274150 (a selective inducible nitric oxide synthase inhibitor) in rheumatoid arthritis. Clin. Exp. Rheumatol. 30, 254–261 (2012).
Hellio le Graverand, M. P. et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis. 72, 187–195 (2013).
Arai, Y. Method for treating cancer by combined use of drugs. PATENTSCOPE [online], (2014).
Flitney, F. W. et al. Antitumor actions of ruthenium(III)-based nitric oxide scavengers and nitric oxide synthase inhibitors. Mol. Cancer Ther. 10, 1571–1580 (2011).
Andrade, S. P., Hart, I. R. & Piper, P. J. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br. J. Pharmacol. 107, 1092–1095 (1992).
Meyer, R. E. et al. Nitric oxide synthase inhibition irreversibly decreases perfusion in the R3230Ac rat mammary adenocarcinoma. Br. J. Cancer 71, 1169–1174 (1995).
Gallo, O. et al. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl Cancer Inst. 90, 587–596 (1998).
Jadeski, L. C. & Lala, P. K. Nitric oxide synthase inhibition by NG-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am. J. Pathol. 155, 1381–1390 (1999).
Kashiwagi, S. et al. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J. Clin. Invest. 115, 1816–1827 (2005).
Lampson, B. L. et al. Targeting eNOS in pancreatic cancer. Cancer Res. 72, 4472–4482 (2012).
Kashiwagi, S. et al. Perivascular nitric oxide gradients normalize tumor vasculature. Nat. Med. 14, 255–257 (2008).
Yang, Z. et al. Targeting nitric oxide signaling with nNOS inhibitors as a novel strategy for the therapy and prevention of human melanoma. Antioxid. Redox Signal. 19, 433–447 (2013).
Ng, Q. S. et al. Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: a phase I study. Lancet Oncol. 8, 111–118 (2007). This study shows that NOS inhibition suppresses tumour blood flow in patients with cancer.
Maines, M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 2, 2557–2568 (1988).
Otterbein, L. E. & Choi, A. M. Heme oxygenase: colors of defense against cellular stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1029–L1037 (2000).
Ryter, S. W. & Otterbein, L. E. Carbon monoxide in biology and medicine. Bioessays 26, 270–280 (2004).
Wu, L. & Wang, R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 57, 585–630 (2005).
Motterlini, R. & Otterbein, L. E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 9, 728–743 (2010).
Zuckerbraun, B. S. et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 21, 1099–1106 (2007). This study connects the mitochondria-inhibiting effect of CO to CO-mediated cytoprotective signalling in vitro.
Chin, B. Y. et al. Hypoxia-inducible factor 1α stabilization by carbon monoxide results in cytoprotective preconditioning. Proc. Natl Acad. Sci. USA 104, 5109–5114 (2007).
Schacter, B. A. & Kurz, P. Alterations in hepatic and splenic microsomal electron transport system components, drug metabolism, heme oxygenase activity, and cytochrome P-450 turnover in Murphy-Sturm lymphosarcoma-bearing rats. Cancer Res. 42, 3557–3564 (1982).
Was, H., Dulak, J. & Jozkowicz, A. Heme oxygenase-1 in tumor biology and therapy. Curr. Drug Targets 11, 1551–1570 (2010).
Berberat, P. O. et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin. Cancer Res. 11, 3790–3798 (2005).
Sass, G. et al. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int. J. Cancer 123, 1269–1277 (2008). This study shows that silencing of HO1 suppresses tumour growth in vivo.
Alaoui-Jamali, M. A. et al. A novel experimental heme oxygenase-1-targeted therapy for hormone-refractory prostate cancer. Cancer Res. 69, 8017–8024 (2009).
Li, Y. et al. PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J. Pathol. 224, 90–100 (2011).
Sahoo, S. K. et al. Pegylated zinc protoporphyrin: a water-soluble heme oxygenase inhibitor with tumor-targeting capacity. Bioconjug. Chem. 13, 1031–1038 (2002).
Fang, J. et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 63, 3567–3574 (2003). This study shows that inhibition of HO1 suppresses tumour growth in vivo.
Hirai, K., Sasahira, T., Ohmori, H., Fujii, K. & Kuniyasu, H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int. J. Cancer 120, 500–505 (2007).
Nowis, D. et al. Zinc protoporphyrin IX, a heme oxygenase-1 inhibitor, demonstrates potent antitumor effects but is unable to potentiate antitumor effects of chemotherapeutics in mice. BMC Cancer 8, 197 (2008).
Miyake, M. et al. Heme oxygenase-1 promotes angiogenesis in urothelial carcinoma of the urinary bladder. Oncol. Rep. 25, 653–660 (2011).
Deng, R. et al. Inhibition of tumor growth and alteration of associated macrophage cell type by an HO-1 inhibitor in breast carcinoma-bearing mice. Oncol. Res. 20, 473–482 (2013).
Gueron, G. et al. Critical role of endogenous heme oxygenase 1 as a tuner of the invasive potential of prostate cancer cells. Mol. Cancer Res. 7, 1745–1755 (2009).
Zou, C. et al. Heme oxygenase-1: a molecular brake on hepatocellular carcinoma cell migration. Carcinogenesis 32, 1840–1848 (2011).
Kappas, A., Drummond, G. S., Manola, T., Petmezaki, S. & Valaes, T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics 81, 485–497 (1988).
Dover, S. B., Moore, M. R., Fitzsimmons, E. J., Graham, A. & McColl, K. E. Tin protoporphyrin prolongs the biochemical remission produced by heme arginate in acute hepatic porphyria. Gastroenterology 105, 500–506 (1993).
Kappas, A., Drummond, G. S., Henschke, C. & Valaes, T. Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics 95, 468–474 (1995).
Nakamura, H., Fang, J., Gahininath, B., Tsukigawa, K. & Maeda, H. Intracellular uptake and behavior of two types zinc protoporphyrin (ZnPP) micelles, SMA-ZnPP and PEG-ZnPP as anticancer agents; unique intracellular disintegration of SMA micelles. J. Control. Release 155, 367–375 (2011).
Tsukigawa, K., Nakamura, H., Fang, J., Otagiri, M. & Maeda, H. Effect of different chemical bonds in pegylation of zinc protoporphyrin that affects drug release, intracellular uptake, and therapeutic effect in the tumor. Eur. J. Pharm. Biopharm. 89, 259–270 (2015).
Pittalà, V., Salerno, L., Romeo, G., Modica, M. N. & Siracusa, M. A. A focus on heme oxygenase-1 (HO-1) inhibitors. Curr. Med. Chem. 20, 3711–3732 (2013).
Bergstraesser, C. et al. Inhibition of VCAM-1 expression in endothelial cells by CORM-3: the role of the ubiquitin-proteasome system, 38, and mitochondrial respiration. Free Radic. Biol. Med. 52, 794–802 (2012).
Schwer, C. I. et al. Carbon monoxide releasing molecule-2 CORM-2 represses global protein synthesis by inhibition of eukaryotic elongation factor eEF2. Int. J. Biochem. Cell Biol. 45, 201–212 (2013).
Wegiel, B. et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 73, 7009–7021 (2013). This study demonstrates the inhibitory effects of CO inhalation therapy on tumour growth in vivo.
Long, R., Salouage, I., Berdeaux, A., Motterlini, R. & Morin, D. CORM-3, a water soluble CO-releasing molecule, uncouples mitochondrial respiration via interaction with the phosphate carrier. Biochim. Biophys. Acta 1837, 201–209 (2014).
Vítek, L. et al. Antiproliferative effects of carbon monoxide on pancreatic cancer. Dig. Liver Dis. 46, 369–375 (2014). This study demonstrates the inhibitory effects of CO inhalation therapy or parenteral CORM therapy on tumour growth in vivo.
Stamellou, E. et al. Different design of enzyme-triggered CO-releasing molecules (ET-CORMs) reveals quantitative differences in biological activities in terms of toxicity and inflammation. Redox Biol. 2, 739–748 (2014). This paper introduces the concept of enzyme-triggered CORM release.
Lee, W. Y. et al. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol. Appl. Pharmacol. 274, 55–62 (2014).
Foresti, R., Bani-Hani, M. G. & Motterlini, R. Use of carbon monoxide as a therapeutic agent: promises and challenges. Int. Care Med. 34, 649–658 (2008).
Otterbein, L. E. Quoth the Raven: carbon monoxide and nothing more. Med. Gas Res. 3, 7 (2013).
Plummer, A. L. The carbon monoxide diffusing capacity: clinical implications, coding, and documentation. Chest 134, 663–667 (2008).
Vesely, A. E. et al. The effects of carbon monoxide on respiratory chemoreflexes in humans. Environ. Res. 94, 227–233 (2004).
Mayr, F. B. et al. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am. J. Respir. Crit. Care Med. 171, 354–360 (2005).
Resch, H., Zawinka, C., Weigert, G., Schmetterer, L. & Garhöfer, G. Inhaled carbon monoxide increases retinal and choroidal blood flow in healthy humans. Invest. Ophthalmol. Vis. Sci. 46, 4275–4280 (2005).
Bathoorn, E. et al. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur. Respir. J. 30, 1131–1137 (2007).
Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 6, 917–935 (2007).
Whiteman, M., Le Trionnaire, S., Chopra, M., Fox, B. & Whatmore, J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin. Sci. (Lond) 121, 459–488 (2011).
Kimura, H. Hydrogen sulfide: its production, release and functions. Amino Acids 41, 113–121 (2011).
Wallace, J. L., Ferraz, J. G. & Muscara, M. N. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid. Redox Signal. 17, 58–67 (2012).
Wang, R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 92, 791–896 (2012).
Shibuya, N. et al. A novel pathway for the production of hydrogen sulfide from d-cysteine in mammalian cells. Nat. Commun. 4, 1366 (2013).
Asimakopoulou, A. et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 169, 922–932 (2013).
Papapetropoulos, A. et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl Acad. Sci. USA 106, 21972–21977 (2009).
Coletta, C. et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl Acad. Sci. USA 109, 9161–9166 (2012). This study shows evidence for cooperative signalling between NO and H 2 S in the control of angiogenesis and vascular relaxation.
Szabo, C. et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 171, 2099–2122 (2014).
Szabo, C. et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl Acad. Sci. USA 110, 12474–12479 (2013). This study shows that silencing CBS or inhibiting CBS suppresses colon cancer bioenergetics and growth in vivo.
Bhattacharyya, S. et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 8, e79167 (2013). This study shows that silencing CBS or inhibiting CBS suppresses ovarian cancer bioenergetics and growth in vivo.
Guo, H. et al. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology 79, 483.e1–483.e5 (2012).
Sen, S. et al. Role of cystathionine β-synthase in human breast cancer. Free Radic. Biol. Med. 86, 228–238 (2015).
Chao, C. et al. Cystathionine-β-synthetase inhibition in combination with standard-chemotherapy decreases colorectal cancer metastasis to the liver. Nitric Oxide 39, S21–S22 (2014).
Takano, N. et al. Decreased expression of cystathionine β-synthase promotes glioma tumorigenesis. Mol. Cancer Res. 12, 1398–1406 (2014).
Hellmich, M. R., Coletta, C., Chao, C. & Szabo, C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 22, 424–448 (2015).
Panza, E. et al. Role of the cystathionine γ lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 28, 61–72 (2015).
De Vos, J. et al. Comparison of gene expression profiling between malignant and normal plasma cells with oligonucleotide arrays. Oncogene 21, 6848–6857 (2002).
Hansel, D. E. et al. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am. J. Pathol. 163, 217–229 (2003).
Zhang, W. et al. Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res. 65, 1554–1560 (2005).
Jurkowska, H., Placha, W., Nagahara, N. & Wróbel, M. The expression and activity of cystathionine-γ-lyase and 3-mercaptopyruvate sulfurtransferase in human neoplastic cell lines. Amino Acids 41, 151–158 (2011).
Szczesny, B., Brunyanszki, A., Hellmich, M. R. & Szabo, C. Hydrogen sulfide in lung adenocarcinoma: a tumor cell survival factor, an enhancer of mitochondrial DNA repair and a cellular bioenergetic stimulator. Nitric Oxide 47, S46 (2015).
Fan, K. et al. Wnt/β-catenin signaling induces the transcription of cystathionine-γ-lyase, a stimulator of tumor in colon cancer. Cell Signal. 26, 2801–2808 (2014).
Zatarain, J. R. et al. H2S inhibition of cystathionine-β-synthase (CBS) using a novel prodrug decreases colorectal cancer xenograft growth with less toxicity than aminooxyacetic acid (AOAA). Gastroenterology 148, S950 (2015).
Perry, T. L. et al. Failure of aminooxyacetic acid therapy in Huntington disease. Neurology 30, 772–775 (1980).
Guth, P. S. et al. Evaluation of amino-oxyacetic acid as a palliative in tinnitus. Ann. Otol. Rhinol. Laryngol. 99, 74–79 (1990).
Li, L. et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117, 2351–2360 (2008).
Han, Y. et al. Hydrogen sulfide inhibits abnormal proliferation of lymphocytes via AKT/GSK3β signal pathway in systemic lupus erythematosus patients. Cell. Physiol. Biochem. 31, 795–804 (2013).
Lee, Z. W. et al. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Br. J. Pharmacol. 171, 4322–4336 (2014).
Lee, Z. W. et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE 6, e21077 (2011). This study demonstrates the inhibitory effects of a slow-releasing H 2 S donor on tumour growth in vivo.
Lu, S., Gao, Y., Huang, X. & Wang, X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int. J. Oncol. 44, 1259–1267 (2014).
Benavides, G. A. et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl Acad. Sci. USA 104, 17977–17982 (2007).
Insko, M. A., Deckwerth, T. L., Hill, P., Toombs, C. F. & Szabo, C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br. J. Pharmacol. 157, 944–951 (2009).
Predmore, B. L. et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol. 302, H2410–H2418 (2012).
Yi, L. & Su, Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem. Toxicol. 57, 362–370 (2013).
Singh, S. V. et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 68, 9503–9511 (2008).
Wu, P. P. et al. Diallyl trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft model in vivo. Phytomedicine 18, 672–676 (2011).
Li, W. et al. Diallyl trisulfide induces apoptosis and inhibits proliferation of A549 cells in vitro and in vivo. Acta Biochim. Biophys. Sin. (Shanghai) 44, 577–583 (2012).
Wallace, G. C. et al. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J. Neurooncol. 114, 43–50 (2013).
Ma, K. et al. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS ONE 6, e20525 (2011).
Chattopadhyay, M. et al. Hydrogen sulfide-releasing aspirin suppresses NF-κB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem. Pharmacol. 83, 723–732 (2012).
Kashfi, K. & Olson, K. R. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem. Pharmacol. 85, 689–703 (2013).
Gemici, B. et al. H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide 46, 25–31 (2015).
Szabo, C. Gaseotransmitters: new frontiers for translational science. Sci. Transl. Med. 2, 59ps54 (2010).
Chattopadhyay, M., Kodela, R., Olson, K. R. & Kashfi, K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem. Biophys. Res. Commun. 419, 523–528 (2012).
Maksimovic-Ivanic, D. et al. Anticancer properties of the novel nitric oxide-donating compound (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid–nitric oxide in vitro and in vivo. Mol. Cancer Ther. 7, 510–520 (2008).
Mijatovic, S. et al. Induction of caspase-independent apoptotic-like cell death of mouse mammary tumor TA3Ha cells in vitro and reduction of their lethality in vivo by the novel chemotherapeutic agent GIT-27NO. Free Radic. Biol. Med. 48, 1090–1099 (2010).
Donia, M. et al. In vitro and in vivo anticancer action of Saquinavir-NO, a novel nitric oxide-derivative of the protease inhibitor saquinavir, on hormone resistant prostate cancer cells. Cell Cycle 10, 492–499 (2011).
Donia, M. et al. Unique antineoplastic profile of Saquinavir-NO, a novel NO-derivative of the protease inhibitor Saquinavir, on the in vitro and in vivo tumor formation of A375 human melanoma cells. Oncol. Rep. 28, 682–688 (2012).
Elsheikh, W., Blackler, R. W., Flannigan, K. L. & Wallace, J. L. Enhanced chemopreventive effects of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346) in experimental colorectal cancer. Nitric Oxide 41, 131–137 (2014).
Moriyama, A. et al. Plasma nitrite/nitrate concentrations as a tumor marker for hepatocellular carcinoma. Clin. Chim. Acta 296, 181–191 (2000).
Chan, H. P., Lewis, C. & Thomas, P. S. Exhaled breath analysis: novel approach for early detection of lung cancer. Lung Cancer 63, 164–168 (2009). This paper presents an approach of measuring patient-exhaled levels of NO for the detection of lung cancer.
Yin, H., Fang, J., Liao, L., Maeda, H. & Su, Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. BMC Cancer 14, 436 (2014). This study indicates there are increased levels of the CO metabolite carboxyhaemoglobin in the circulation of patients with cancer.
Bonavida, B. & Baritaki, S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: downregulation of the NF-kB/Snail/YY1/RKIP circuitry. Nitric Oxide 24, 1–7 (2011).
Bhowmick, R. & Girotti, A. W. Cytoprotective signaling associated with nitric oxide upregulation in tumor cells subjected to photodynamic therapy-like oxidative stress. Free Radic. Biol. Med. 57, 39–48 (2013).
Furfaro, A. L. et al. HO-1 up-regulation: a key point in high-risk neuroblastoma resistance to bortezomib. Biochim. Biophys. Acta 1842, 613–622 (2014).
Sanokawa-Akakura, R., Ostrakhovitch, E. A., Akakura, S., Goodwin, S. & Tabibzadeh, S. A H2S-Nampt dependent energetic circuit is critical to survival and cytoprotection from damage in cancer cells. PLoS ONE 9, e108537 (2014).
Ostrakhovitch, E. A., Akakura, S., Sanokawa-Akakura, R., Goodwin, S. & Tabibzadeh, S. Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp. Cell Res. 330, 135–150 (2015).
Eyler, C. E. et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell 146, 53–66 (2011).
Herrmann, H. et al. The Hsp32 inhibitors SMA-ZnPP and PEG-ZnPP exert major growth-inhibitory effects on D34+/CD38+ and CD34+/CD38− AML progenitor cells. Curr. Cancer Drug Targets 12, 51–63 (2012).
Kim, R. K. et al. Fractionated radiation-induced nitric oxide promotes expansion of glioma stem-like cells. Cancer Sci. 104, 1172–1177 (2013).
Rao, C. V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res. 555, 107–119 (2004).
Bonavida, B., Khineche, S., Huerta-Yepez, S. & Garbán, H. Therapeutic potential of nitric oxide in cancer. Drug Resist. Updat. 9, 157–173 (2006).
Burke, A. J., Sullivan, F. J., Giles, F. J. & Glynn, S. A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 34, 503–512 (2013).
Grochot-Przeczek, A., Dulak, J. & Jozkowicz, A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin. Sci. (Lond). 122, 93–103 (2012).
Carbonero, F., Benefiel, A. C. & Gaskins, H. R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 9, 504–518 (2012).
Kilbourn, R. G. et al. Inhibition of interleukin-1-α-induced nitric oxide synthase in vascular smooth muscle and full reversal of interleukin-1-α-induced hypotension by Nω-amino-L-arginine. J. Natl Cancer Inst. 84, 1008–1016 (1992).
Kilbourn, R. G., Szabo, C. & Traber, D. L. Beneficial versus detrimental effects of nitric oxide synthase inhibitors in circulatory shock: lessons learned from experimental and clinical studies. Shock 7, 235–246 (1997).
Kondapaneni, M., McGregor, J. R., Salvemini, D., Laubach, V. E. & Samlowski, W. E. Inducible nitric oxide synthase (iNOS) is not required for IL-2-induced hypotension and vascular leak syndrome in mice. J. Immunother. 31, 325–333 (2008).
Samlowski, W. E. et al. Endothelial nitric oxide synthase is a key mediator of interleukin-2-induced hypotension and vascular leak syndrome. J. Immunother. 34, 419–427 (2011).
Maybauer, D. M. et al. Lung-protective effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 in interleukin-2 induced toxicity. Biochem. Biophys. Res. Commun. 377, 786–791 (2008).
Kilbourn, R. G., Fonseca, G. A., Trissel, L. A. & Griffith, O. W. Strategies to reduce side effects of interleukin-2: evaluation of the antihypotensive agent NG-monomethyl-L-arginine. Cancer J. Sci. Am. 6, S21–S30 (2000). This study shows that inhibition of NOS production by the non-isoform-selective NOS inhibitor L -NMA reduces the hypotensive side effects of IL-2 during the immunotherapy of patients with cancer.
Ashburn, T. T. & Thor, K. B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683 (2004).
Blatt, J. & Corey, S. J. Drug repurposing in pediatrics and pediatric hematology oncology. Drug Discov. Today 18, 4–10 (2013).
Stenvang, J. et al. Biomarker-guided repurposing of chemotherapeutic drugs for cancer therapy: a novel strategy in drug development. Front. Oncol. 3, 313 (2013).
Quinn, B. J. et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol. Metab. 24, 469–480 (2013).
Jordan, B. F. et al. Nitric oxide as a radiosensitizer: evidence for an intrinsic role in addition to its effect on oxygen delivery and consumption. Int. J. Cancer 109, 768–773 (2004).
Oronsky, B. T., Knox, S. J. & Scicinski, J. J. Is nitric oxide (NO) the last word in radiosensitization? A review. Transl. Oncol. 5, 66–71 (2012).
Arrieta, O. et al. Phase II study. Concurrent chemotherapy and radiotherapy with nitroglycerin in locally advanced non-small cell lung cancer. Radiother. Oncol. 111, 311–315 (2014).
Reid, T. et al. Two case reports of resensitization to previous chemotherapy with the novel hypoxia-activated hypomethylating anticancer agent RRx-001 in metastatic colorectal cancer patients. Case Rep. Oncol. 7, 79–85 (2014).
Jiang, H. et al. Activated macrophages as a novel determinant of tumor cell radioresponse: the role of nitric oxide-mediated inhibition of cellular respiration and oxygen sparing. Int. J. Radiat. Oncol. Biol. Phys. 76, 1520–1527 (2010).
de Bono, J. S. et al. Phase I study of ONO-4007, a synthetic analogue of the lipid A moiety of bacterial lipopolysaccharide. Clin. Cancer Res. 6, 397–405 (2000).
Acknowledgements
The author's research in the field of H2S and cancer is supported by a grant from the US National Institutes of Health (NIH; R01CA175803) and the US Cancer Prevention Research Institute of Texas (CPRIT; DP150074).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author is a principal and a shareholder of CBS Therapeutics Inc., a start-up company involved in the research and development of CBS inhibitors for cancer therapy.
Glossary
- Peroxynitrite
-
A short-lived cytotoxic oxidant species that is the product of the diffusion- controlled reaction between nitric oxide (NO) and a superoxide radical (O2−).
- Bacillus Calmette–Guérin
-
A live attenuated strain of Mycobacterium bovis that is a US-approved therapy for in situ bladder carcinoma.
- 3-methylcholanthrene
-
The most common isomer of a highly carcinogenic polycyclic aromatic hydrocarbon — its topical administration in mice is often used as an experimental cancer model.
- Secretagogue
-
A biological substance that induces the secretion of another substance. For example, angiotensin II is a secretagogue for aldosterone.
- Glutathione-S-transferase
-
(GST). A soluble protein with a molecular mass of ~50 kDa. GSTs represent a major group of detoxification enzymes and catalyse the conjugation of the reduced form of glutathione (GSH) to various cellular substrates.
- Multi-arm polymeric nanocarriers
-
Branched, globular, nanoscale materials exhibiting a large surface area. They are commonly used for targeted drug delivery.
- Prodrugs
-
Inactive precursors of active therapeutic agents. The conversion from the inactive to the active form occurs through normal metabolic processes, often involving the hydrolysis of an ester group.
- Chorioallantoic membrane model
-
A common experimental model in which melanoma cells are grown on chick chorioallantoic membranes (CAMs). It is a model with a substantial angiogenesis component.
- Epithelial–mesenchymal transition
-
(EMT). A process by which epithelial cells lose their cell polarity and cell–cell adhesion and assume a migratory and invasive phenotype.
- Protoporphyrins
-
(PPs). Tetrapyrroles containing four methyl side chains, two propionic acid side chains and two vinyl side chains. The iron complex of PPs occurs in a number of proteins, including haemoglobin, myoglobin and several electron transport proteins of the mitochondrial respiratory chain.
- Transgenic adenocarcinoma mouse prostate cancer model
-
(TRAMP cancer model). One of the most well-known prostate cancer mouse models. The expression of both the large and small SV40 tumour antigens is regulated by the prostate-specific rat probasin promoter.
- Theranostic approach
-
Approaches that incorporate the development of molecular diagnostic tests in combination with targeted therapeutics. These approaches are integral to the personalized medicine concept. Also known as 'theranostics'.
Rights and permissions
About this article
Cite this article
Szabo, C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat Rev Drug Discov 15, 185–203 (2016). https://doi.org/10.1038/nrd.2015.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2015.1
This article is cited by
-
Pan-inhibition of the three H2S synthesizing enzymes restrains tumor progression and immunosuppression in breast cancer
Cancer Cell International (2024)
-
Sex differences in colonic gene expression and fecal microbiota composition in a mouse model of obesity-associated colorectal cancer
Scientific Reports (2024)
-
Radiolysis of myoglobin concentrated gels by protons: specific changes in secondary structure and production of carbon monoxide
Scientific Reports (2024)
-
Local delivery of gaseous signaling molecules for orthopedic disease therapy
Journal of Nanobiotechnology (2023)
-
Gas therapy potentiates aggregation-induced emission luminogen-based photoimmunotherapy of poorly immunogenic tumors through cGAS-STING pathway activation
Nature Communications (2023)