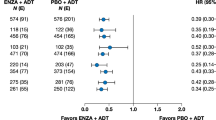
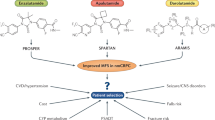
Key Points
-
The increasing number of both approved and experimental therapies available mandates the use of new trial designs for patients with noncastrate prostate cancer, which provide expedited readouts of efficacy
-
Trials based on time-to-event end points, such as progression to metastatic disease and overall survival, require large numbers of patients with long follow-up durations, and might provide inconclusive results owing to use of post-protocol interventions
-
To accelerate progress, a multi-arm, multistage, multimodality trial platform involving delivery of systemic therapy, radiotherapy to detectable metastases, and radical surgery was developed that enables new arms to be added at any time
-
The objective is to eliminate all disease using binary quantitative end points that occur early and solely reflect the effects of treatment, such as pathological complete response and undetectable serum prostate-specific antigen levels after testosterone recovery
Abstract
The unprecedented progress in the treatment of metastatic castration-resistant prostate cancer is only beginning to be realized in patients with noncastrate disease. This slow progress in part reflects the use of trial objectives focused on time-to-event end points, such as time to metastasis and overall survival, which require long follow-up durations and large sample sizes, and has been further delayed by the use of approved therapies that are effective at the time of progression. Our central hypotheses are that progress can be accelerated, and that outcomes can be improved by shifting trial objectives to response measures occurring early that solely reflect the effects of the treatment. To test these hypotheses, a continuously enrolling multi-arm, multi-stage randomized trial design, analogous to that used in the STAMPEDE trial, has been developed. Eligibility is focused on patients with incurable disease or those with a high risk of death with any form of monotherapy alone. The primary objective is to eliminate all disease using a multimodality treatment strategy. End points include pathological complete response and an undetectable level of serum prostate-specific antigen, with recovery of serum testosterone levels. Both are binary, objective, and provide an early, quantitative indication of efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
08 November 2017
In the version of this Review published online ahead of print, The Acknowledgements section in the online and PDF versions of this manuscript originally contained the text "The authors gratefully acknowledge financial support from the Prostate Cancer Clinical Trials Consortium of the Prostate Cancer Foundation, and a SPORE Center Grant to the Sidney Kimmel Center for Prostate and Urologic Cancers." This was incorrect and has been corrected to "The authors gratefully acknowledge financial support from the Department of Defense Prostate Cancer Research Program (PC121111 and PC131984), the NIH/NCI (Cancer Center Support Grant P30-CA008748, P50-CA92629 SPORE in Prostate Cancer), the Prostate Cancer Foundation, and the Sidney Kimmel Center for Prostate and Urologic Cancers” in the online and PDF versions of this manuscript.
References
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Abida, W. et al. Genomic characterization of primary and metastatic prostate cancer (PC) using a targeted next-generation sequencing assay [abstract]. J. Clin. Oncol. 34 (Suppl.), 254 (2016).
Scher, H. I. et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 26, 1148–1159 (2008).
[No authors listed.] NOVANTRONE® mitoXANTRONE for injection concentrate. U.S. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019297s030s031lbl.pdf (2008).
Morris, M. J. et al. Correlation between radiographic progression-free survival (rPFS) and overall survival (OS): Results from PREVAIL [abstract]. J. Clin. Oncol. 34 (Suppl.), 182 (2016).
Morris, M. J. et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J. Clin. Oncol. 33, 1356–1363. (2015).
Huggins, C. The Treatment of Cancer of the Prostate (The 1943 Address in Surgery before the Royal College of Physicians and Surgeons of Canada). Can. Med. Assoc. J. 50, 301–307 (1944).
Chang, A. J., Autio, K. A., Roach, M., 3rd, Scher, H. I. High-risk prostate cancer-classification and therapy. Nat. Rev. Clin. Oncol. 11, 308–323 (2014).
Mason, M. D. et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J. Clin. Oncol. 33, 2143–2150 (2015).
Widmark, A. et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 373, 301–308 (2009).
Friedland, D. et al. A phase II trial of docetaxel (Taxotere) in hormone-refractory prostate cancer: correlation of antitumor effect to phosphorylation of Bcl-2. Semin. Oncol. 26, 19–23 (1999).
Picus, J. & Schultz, M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin. Oncol. 26, 14–18 (1999).
Fizazi, K. et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 16, 787–794 (2015).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Sandler, H. M. et al. A phase III protocol of androgen suppression (AS) and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy (CT) with docetaxel and prednisone for localized, high-risk prostate cancer (RTOG 0521) [abstract]. J. Clin. Oncol. 33 (Suppl.), LBA5002 (2015).
Glode, L. M. et al. Adjuvant androgen deprivation (ADT) versus mitoxantrone plus prednisone (MP) plus ADT in high-risk prostate cancer (PCa) patients following radical prostatectomy: A phase III intergroup trial (SWOG S9921) NCT00004124 [abstract]. J. Clin. Oncol. 35 (Suppl.), 2 (2017).
Royce, T. J. et al. Surrogate end points for all-cause mortality in men with localized unfavorable-risk prostate cancer treated with radiation therapy versus radiation therapy plus androgen deprivation therapy: a secondary analysis of a randomized clinical trial. JAMA Oncol. 3, 652–658 (2017).
ICECaP Working Group et al. The development of Intermediate Clinical Endpoints in Cancer of the Prostate (ICECaP). J. Natl Cancer Inst. 107, djv261 (2015).
Xie, W. et al. Metastasis-free survival (MFS) is a surrogate for overall survival (OS) in localized prostate cancer (CaP) [abstract]. Ann. Oncol. 27 (Suppl. 6), 717O (2016).
Pound, C. R. et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281, 1591–1597 (1999).
Yossepowitch, O. et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J. Urol. 178, 493–499 (2007).
Briganti, A. et al. Prediction of outcome following early salvage radiotherapy among patients with biochemical recurrence after radical prostatectomy. Eur. Urol. 66, 479–486 (2014).
Brockman, J. A. et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur. Urol. 67, 1160–1167 (2015).
Katz, M. S. et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J. Clin. Oncol. 21, 483–489 (2003).
Karlin, J. D. et al. Identifying appropriate patients for early salvage radiotherapy after prostatectomy. J. Urol. 190, 1410–1415 (2013).
Shipley, W. U. et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N. Engl. J. Med. 376, 417–428 (2017).
Carrie, C. et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 17, 747–756 (2016).
Crawford, E. D. et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N. Engl. J. Med. 321, 419–424 (1989).
Eisenberger, M. A. et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N. Engl. J. Med. 339, 1036–1042 (1998).
Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355, 1491–1498 (2000).
Bennett, C. L. et al. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: a meta-analysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate Cancer Prostatic Dis. 2, 4–8 (1999).
Bertagna, C., De Gery, A., Hucher, M., Francois, J. P. & Zanirato, J. Efficacy of the combination of nilutamide plus orchidectomy in patients with metastatic prostatic cancer. A meta-analysis of seven randomized double-blind trials (1056 patients). Br. J. Urol. 73, 396–402 (1994).
Caubet, J. F. et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology 49, 71–78 (1997).
Schmitt, B. et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology 57, 727–732 (2001).
Seidenfeld, J. et al. Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid. Rep. Technol. Assess. 4, 1–246 (1999).
Sweeney, C. et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial [abstract]. J. Clin. Oncol. 32, (Suppl.), LBA2 (2014).
Sweeney, C. J. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
Sweeney, C. et al. Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial [abstract]. Ann. Oncol. 27 (Suppl. 6), 720PD (2016).
James, N. D. et al. Docetaxel and/or zoledronic acid for hormone-naïve prostate cancer: first overall survival results from STAMPEDE (NCT00268476) [abstract]. J. Clin. Oncol. 33, (Suppl.), 5001 (2015).
James, N. D. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016).
Gravis, G. et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 14, 149–158 (2013).
Gravis, G. et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur. Urol. 70, 256–262 (2015).
Fizazi, K. et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 377, 352–360 (2017).
James, N. D. et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med. 377, 338–351 (2017).
Taplin, M. E. et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J. Clin. Oncol. 32, 3705–3715 (2014).
O'Shaughnessy, M. J. et al. A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early stage metastatic prostate cancer. Urology 102, 164–172 (2016).
Sternberg, C. N. et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J. Urol. 139, 461–469 (1988).
Hendry, W. F. et al. Metastatic nonseminomatous germ cell tumors of the testis: results of elective and salvage surgery for patients with residual retroperitoneal masses. Cancer 94, 1668–1676 (2002).
Eggener, S. E. et al. Pathologic findings and clinical outcome of patients undergoing retroperitoneal lymph node dissection after multiple chemotherapy regimens for metastatic testicular germ cell tumors. Cancer 109, 528–535 (2007).
Symmans, W. F. et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J. Clin. Oncol. 35, 1049–1060 (2017).
Berruti, A. et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J. Clin. Oncol. 32, 3883–3891 (2014).
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Shore, N. D. et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 17, 153–163 (2016).
Penson, D. F. et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J. Clin. Oncol. 34, 2098–2106 (2016).
Scher, H. I. et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010).
Symmans, W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–4422 (2007).
Crook, J. M. et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N. Engl. J. Med. 367, 895–903 (2012).
Hussain, M. et al. Intermittent versus continuous androgen deprivation in prostate cancer. N. Engl. J. Med. 368, 1314–1325 (2013).
Rathkopf, D. et al. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J. Clin. Oncol. 26, 2959–2965 (2008).
Morris, M. J. et al. Efficacy analysis of a phase III study of androgen deprivation therapy (ADT) +/- docetaxel (D) for men with biochemical relapse (BCR) after prostatectomy [abstract]. J. Clin. Oncol. 33 (Suppl.), 5011 (2015).
James, N. D. et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur. Urol. 67, 1028–1038 (2015).
Hussain, M. et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J. Clin. Oncol. 24, 3984–3990 (2006).
Heidenreich, A., Pfister, D. & Porres, D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J. Urol. 193, 832–838 (2015).
Sooriakumaran, P. et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur. Urol. 69, 788–794 (2016).
Antwi, S. & Everson, T. M. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: a population-based, propensity score analysis. Cancer Epidemiol. 38, 435–441 (2014).
Culp, S. H., Schellhammer, P. F. & Williams, M. B. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur. Urol. 65, 1058–1066 (2014).
Rusthoven, C. G. et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J. Clin. Oncol. 34, 2835–2842 (2016).
Moschini, M., Soria, F., Briganti, A. & Shariat, S. F. The impact of local treatment of the primary tumor site in node positive and metastatic prostate cancer patients. Prostate Cancer Prostatic Dis. 20, 7–11 (2017).
Ost, P. et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur. Urol. 69, 9–12 (2016).
Seung, S. K. et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) practice guideline for the performance of stereotactic radiosurgery (SRS). Am. J. Clin. Oncol. 36, 310–315 (2013).
Ost, P. et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur. Urol. 67, 852–863 (2015).
Berkovic, P. et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin. Genitourin. Cancer 11, 27–32 (2013).
James, N. D. et al. STAMPEDE: Systemic Therapy for Advancing or Metastatic Prostate Cancer — a multi-arm multi-stage randomised controlled trial. Clin. Oncol. 20, 577–581 (2008).
James, N. D. et al. Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multi-arm, multistage randomized controlled trial. BJU Int. 103, 464–469 (2008).
Parmar, M. K. et al. Speeding up the evaluation of new agents in cancer. J. Natl Cancer Inst. 100, 1204–1214 (2008).
Sydes, M. R. et al. Flexible trial design in practice — stopping arms for lack-of-benefit and adding research arms mid-trial in STAMPEDE: a multi-arm multi-stage randomized controlled trial. Trials 13, 168 (2012).
Epstein, J. I. et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur. Urol. 69, 428–435 (2016).
Den, R. B. et al. Decipher correlation patterns post prostatectomy: initial experience from 2342 prospective patients. Prostate Cancer Prostatic Dis. 19, 374–379 (2016).
Cullen, J. et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur. Urol. 68, 123–131 (2015).
Feng, F. Y. et al. Luminal and basal subtyping of prostate cancer [abstract]. J. Clin. Oncol. 35 (Suppl.), 3 (2017).
Cooperberg, M. R. et al. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur. Urol. 67, 326–333 (2015).
Klein, E. A. et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur. Urol. 67, 778–786 (2015).
Ross, A. E. et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur. Urol. 69, 157–165 (2016).
Touijer, K. A., Mazzola, C. R., Sjoberg, D. D., Scardino, P. T. & Eastham, J. A. Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen-deprivation therapy. Eur. Urol. 65, 20–25 (2014).
Evangelista, L., Guttilla, A., Zattoni, F., Muzzio, P. C. & Zattoni, F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur. Urol. 63, 1040–1048 (2013).
Hovels, A. M. et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol 63, 387–395 (2008).
Maurer, T. et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J. Urol. 195, 1436–1443 (2016).
Kulshrestha, R. K., Vinjamuri, S., England, A., Nightingale, J. & Hogg, P. The role of 18F-sodium fluoride PET/CT bone scans in the diagnosis of metastatic bone disease from breast and prostate cancer. J. Nucl. Med. Technol. 44, 217–222 (2016).
Vargas, H. A. et al. Bone metastases in castration-resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology 271, 220–229 (2014).
Robertson, N. L. et al. Combined whole-body and multi-parametric prostate MRI as a single-step approach for the simultaneous assessment of local recurrence and metastatic disease after radical prostatectomy. J. Urol. 198, 65–70 (2017).
Gillessen, S. et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur. Urol. http://dx.doi.org/10.1016/j.eururo.2017.06.002 (2017).
Kim, M. Y. et al. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326 (2009).
Abdollah, F. et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J. Clin. Oncol. 32, 3939–3947 (2014).
Bolla, M. et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380, 2018–2027 (2012).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. U.S. Food and Drug Administration http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM305501.pdf (2014)
Abida, W. et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may impact clinical decision making. JCO Precis. Oncol. http://dx.doi.org/10.1200/PO.17.00029 (2017).
Jones, C. U. et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 365, 107–118 (2011).
Warde, P. et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 378, 2104–2111 (2011).
Labrie, F. et al. Neoadjuvant hormonal therapy: the Canadian experience. Urology 49, 56–64 (1997).
van der Kwast, T. H. et al. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: three versus six months of endocrine therapy. Urology 53, 523–529 (1999).
Gleave, M. E. et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J. Urol. 166, 500–507 (2001).
Klotz, L. H. et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J. Urol. 170, 791–794 (2003).
Mostaghel, E. A. et al. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J. Clin. Oncol. 32, 229–237 (2014).
Montgomery, B. et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin. Cancer Res. 23, 2169–2176 (2017).
Efstathiou, E. et al. Neoadjuvant enzalutamide (ENZA) and abiraterone acetate (AA) plus leuprolide acetate (LHRHa) versus AA+ LHRHa in localized high-risk prostate cancer (LHRPC) [abstract]. J. Clin. Oncol. 34 (Suppl.), 5002 (2016).
National Comprehensive Cancer Network. Prostate Cancer Guidelines (Version 2.2017). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. (2017).
Thompson, I. et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J. Urol. 177, 2106–2131 (2007).
Mottet, N. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 71, 618–629 (2017).
Roach, M. et al. Four prognostic groups predict long-term survival from prostate cancer following radiotherapy alone on Radiation Therapy Oncology Group clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 47, 609–615 (2000).
Narang, A. K. et al. Very high-risk localized prostate cancer: outcomes following definitive radiation. Int. J. Radiat. Oncol. Biol. Phys. 94, 254–262 (2016)
Sundi, D. et al. Identification of men with the highest risk of early disease recurrence after radical prostatectomy. Prostate 74, 628–636 (2014).
Joniau, S. et al. Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study. Eur. Urol. 67, 157–164 (2015).
Cooperberg, M. R. et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J. Urol. 173, 1938–1942 (2005).
Stephenson, A. J. et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J. Clin. Oncol. 27, 4300–4305 (2009).
Acknowledgements
The authors gratefully acknowledge financial support from the Department of Defense Prostate Cancer Research Program (PC121111 and PC131984), the NIH/NCI (Cancer Center Support Grant P30-CA008748, P50-CA92629 SPORE in Prostate Cancer), the Prostate Cancer Foundation, and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
H.I.S. declares that he is a member of the board of directors of Asterias Biotherapeutics, has served as a compensated consultant of Blue Earth Diagnostics, Sanofi Aventis, and WCG Oncology, and has served as an uncompensated consultant of Ferring Pharmaceuticals, Janssen Research & Development, LLC and Medivation. M.Y.T., M.J.O., S.M.M. and H.A.V. declare no competing interests.
Rights and permissions
About this article
Cite this article
Teo, M., O'Shaughnessy, M., McBride, S. et al. Drug development for noncastrate prostate cancer in a changed therapeutic landscape. Nat Rev Clin Oncol 15, 168–182 (2018). https://doi.org/10.1038/nrclinonc.2017.160
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.160
This article is cited by
-
A first-in-class HBO1 inhibitor WM-3835 inhibits castration-resistant prostate cancer cell growth in vitro and in vivo
Cell Death & Disease (2023)
-
GNE-493 inhibits prostate cancer cell growth via Akt-mTOR-dependent and -independent mechanisms
Cell Death Discovery (2022)
-
Prostate cancer
Nature Reviews Disease Primers (2021)