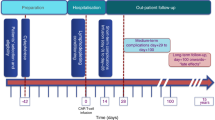
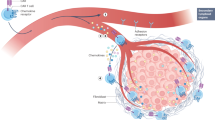
Key Points
-
New treatments are needed for patients with chemotherapy-refractory or multiply-relapsed lymphoma
-
Chimeric antigen receptor (CAR) T cells targeting CD19 have demonstrated efficacy in multiple subtypes of B-cell lymphoma, with activity seen in patients with chemotherapy-refractory lymphoma; durable remissions are possible
-
Multicentre clinical trials have demonstrated that centralized CAR-T-cell processing is feasible, and response rates in early studies of centrally manufactured CAR-T-cell therapies are similar to those reported in single-centre studies
-
CARs targeting novel antigens, such as CD20, CD22, CD30 and κ light chains, are in development and will extend the applicability of CAR-T-cell therapy to patients with Hodgkin lymphoma, T-cell lymphoma, or CD19-negative B-cell lymphoma
-
Cytokine-release syndrome and neurological toxicity are severe adverse events commonly associated with CAR-T-cell therapies for lymphoma, and reducing the risk of such toxicities is a major avenue for improving CAR-T-cell therapies
-
CAR-T-cell therapy is likely to become safer and more effective, and will probably become a standard treatment option for patients with relapsed and primary-chemotherapy-refractory lymphoma in the near future
Abstract
New therapies are needed for patients with Hodgkin or non-Hodgkin lymphomas that are resistant to standard therapies. Indeed, unresponsiveness to standard chemotherapy and relapse after autologous stem-cell transplantation are indicators of an especially poor prognosis. Chimeric antigen receptor (CAR) T cells are emerging as a novel treatment modality for these patients. Clinical trial data have demonstrated the potent activity of anti-CD19 CAR T cells against multiple subtypes of B-cell lymphoma, including diffuse large-B-cell lymphoma (DLBCL), follicular lymphoma, mantle-cell lymphoma, and marginal-zone lymphoma. Importantly, anti-CD19 CAR T cells have impressive activity against chemotherapy-refractory lymphoma, inducing durable complete remissions lasting >2 years in some patients with refractory DLBCL. CAR-T-cell therapies are, however, associated with potentially fatal toxicities, including cytokine-release syndrome and neurological toxicities. CAR T cells with novel target antigens, including CD20, CD22, and κ-light chain for B-cell lymphomas, and CD30 for Hodgkin and T-cell lymphomas, are currently being investigated in clinical trials. Centrally manufactured CAR T cells are also being tested in industry-sponsored multicentre clinical trials, and will probably soon become a standard therapy. Herein, we review the clinical efficacy and toxicity of CAR-T-cell therapies for lymphoma, and discuss their limitations and future directions with regard to toxicity management, CAR designs and CAR-T-cell phenotypes, conditioning regimens, and combination therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Cancer Society. About non-hodgkin lymphoma. American Cancer Society https://www.cancer.org/cancer/non-hodgkin-lymphoma/about/key-statistics.html (2017).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Teras, L. R. et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J. Clin. 66, 443–459 (2016).
Coiffier, B. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242 (2002).
Marcus, R. et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J. Clin. Oncol. 26, 4579–4586 (2008).
Forstpointner, R. et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 104, 3064–3071 (2004).
Elstrom, R. L. et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin. Lymphoma Myeloma Leuk. 10, 192–196 (2010).
Martelli, M. et al. Diffuse large B-cell lymphoma. Crit. Rev. Oncol. Hematol. 87, 146–171 (2013).
Van Den Neste, E. et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 52, 216–221 (2017).
Jurinovic, V. et al. Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood 128, 1112–1120 (2016).
Feugier, P. et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J. Clin. Oncol. 23, 4117–4126 (2005).
Philip, T. et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N. Engl. J. Med. 333, 1540–1545 (1995).
Vose, J. M. et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J. Clin. Oncol. 19, 406–413 (2001).
Telio, D. et al. Salvage chemotherapy and autologous stem cell transplant in primary refractory diffuse large B-cell lymphoma: outcomes and prognostic factors. Leuk. Lymphoma 53, 836–841 (2012).
Seshadri, T. et al. Utility of subsequent conventional dose chemotherapy in relapsed/refractory transplant-eligible patients with diffuse large B-cell lymphoma failing platinum-based salvage chemotherapy. Hematology 13, 261–266 (2008).
Nagle, S. J. et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am. J. Hematol. 88, 890–894 (2013).
Martin, P. et al. Patterns of delivery of chemoimmunotherapy to patients with follicular lymphoma in the United States: results of the National LymphoCare Study. Cancer 119, 4129–4136 (2013).
Rummel, M. J. et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381, 1203–1210 (2013).
Flinn, I. W. et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 123, 2944–2952 (2014).
Geisler, C. H. et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br. J. Haematol. 158, 355–362 (2012).
Geisler, C. H. et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 112, 2687–2693 (2008).
Fisher, R. I. et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 24, 4867–4874 (2006).
Goy, A. et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J. Clin. Oncol. 31, 3688–3695 (2013).
Wang, M. L. et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 369, 507–516 (2013).
Campo, E. & Rule, S. Mantle cell lymphoma: evolving management strategies. Blood 125, 48–55 (2015).
Schmitz, N. et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 116, 3418–3425 (2010).
Simon, A. et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br. J. Haematol. 151, 159–166 (2010).
Armitage, J. O., Vose, J. M. & Weisenburger, D. D. Towards understanding the peripheral T-cell lymphomas. Ann. Oncol. 15, 1447–1449 (2004).
Coiffier, B. et al. Peripheral T-cell lymphomas have a worse prognosis than B-cell lymphomas: a prospective study of 361 immunophenotyped patients treated with the LNH-84 regimen. The GELA (Groupe d'Etude des Lymphomes Agressives). Ann. Oncol. 1, 45–50 (1990).
Escalon, M. P. et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer 103, 2091–2098 (2005).
Coiffier, B. et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J. Clin. Oncol. 30, 631–636 (2012).
O'Connor, O. A. et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J. Clin. Oncol. 33, 2492–2499 (2015).
O'Connor, O. A. et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J. Clin. Oncol. 29, 1182–1189 (2011).
Duggan, D. B. et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J. Clin. Oncol. 21, 607–614 (2003).
Engert, A. et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J. Clin. Oncol. 27, 4548–4554 (2009).
Hoskin, P. J. et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin's lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J. Clin. Oncol. 27, 5390–5396 (2009).
Montanari, F. & Diefenbach, C. Relapsed Hodgkin lymphoma: management strategies. Curr. Hematol. Malig. Rep. 9, 284–293 (2014).
Schmitz, N. et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet 359, 2065–2071 (2002).
Saini, K. S. et al. Rituximab in Hodgkin lymphoma: is the target always a hit? Cancer Treat. Rev. 37, 385–390 (2011).
Younes, A. et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J. Clin. Oncol. 30, 2183–2189 (2012).
Chen, R. et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 128, 1562–1566 (2016).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Younes, A. et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 17, 1283–1294 (2016).
Armand, P. et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 34, 3733–3739 (2016).
Timmerman, J. et al. Checkmate 205 update with minimum 12-month follow up: a phase 2 study of nivolumab in patients with relapsed/refractory classical Hodgkin lymphoma [abstract 1100]. ASH https://ash.confex.com/ash/2016/webprogram/Paper91722.html (2016).
Rezvani, A. R. et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J. Clin. Oncol. 26, 211–217 (2008).
Grigg, A. & Ritchie, D. Graft-versus-lymphoma effects: clinical review, policy proposals, and immunobiology. Biol. Blood Marrow Transplant. 10, 579–590 (2004).
Thomson, K. J. et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin's lymphoma. J. Clin. Oncol. 27, 426–432 (2009).
van Kampen, R. J. et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J. Clin. Oncol. 29, 1342–1348 (2011).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
Kochenderfer, J. N. & Rosenberg, S. A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 10, 267–276 (2013).
Sadelain, M., Brentjens, R. & Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 3, 388–398 (2013).
Johnson, L. A. & June, C. H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 27, 38–58 (2017).
Kochenderfer, J. N. et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 32, 689–702 (2009).
Sadelain, M. CAR therapy: the CD19 paradigm. J. Clin. Invest. 125, 3392–3400 (2015).
Gill, S. & June, C. H. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol. Rev. 263, 68–89 (2015).
Jensen, M. C. & Riddell, S. R. Designing chimeric antigen receptors to effectively and safely target tumors. Curr. Opin. Immunol. 33, 9–15 (2015).
Sadelain, M., Brentjens, R. & Riviere, I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 21, 215–223 (2009).
van der Stegen, S. J., Hamieh, M. & Sadelain, M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 14, 499–509 (2015).
Carpenito, C. et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA 106, 3360–3365 (2009).
Sadelain, M., Riviere, I. & Riddell, S. Therapeutic T cell engineering. Nature 545, 423–431 (2017).
Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549 (2015).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl Med. 5, 177ra38 (2013).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl Med. 6, 224ra25 (2014).
Savoldo, B. et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011).
Ramos, C. A. et al. Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J. Clin. Invest. 126, 2588–2596 (2016).
Kochenderfer, J. N. et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 35, 1803–1813 (2017).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl Med. 7, 303ra139 (2015).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Turtle, C. J. et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123–2138 (2016).
Kebriaei, P. et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Invest. 126, 3363–3376 (2016).
Kochenderfer, J. N., Yu, Z., Frasheri, D., Restifo, N. P. & Rosenberg, S. A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116, 3875–3886 (2010).
Gattinoni, L. et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202, 907–912 (2005).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Davila, M. L., Kloss, C. C., Gunset, G. & Sadelain, M. CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS ONE 8, e61338 (2013).
Hwu, P. et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J. Exp. Med. 178, 361–366 (1993).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–286 (2003).
Cooper, L. J. et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood 101, 1637–1644 (2003).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Jensen, M. C. et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 16, 1245–1256 (2010).
Rezvani, A. R. et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br. J. Haematol. 143, 395–403 (2008).
Gisselbrecht, C. et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 28, 4184–4190 (2010).
Turtle, C. J. et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl Med. 8, 355ra116 (2016).
Sommermeyer, D. et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 30, 492–500 (2016).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Schuster, S. J. et al. Sustained remissions following chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas [abstract]. Blood 126, 183 (2015).
Wang, X. et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood 127, 2980–2990 (2016).
Locke, F. L. et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol. Ther. 25, 285–295 (2017).
Locke, F. L. Clinical and biologic covariates of outcomes in ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (r-NHL). J. Clin. Oncol. 35 (Suppl.), abstr. 7512 (2017).
Locke, F. L. et al. Primary results from ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axicel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL). Cancer Res. 77 (13 Suppl.), abstr. CT019 (2017).
Abramson, J. S. et al. CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T-cell product JCAR017 (TRANSCEND NHL 001). J. Clin. Oncol. 35 (Suppl.), abstr. 7513 (2017).
Schuster, S. J. Global pivotal phase 2 trial of the CD19-targeted therapy CTL019 in adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) — an interim analysis. Hematol. Oncol. 35 (Suppl. S2), 27 (2017).
van den Brink, M. R. et al. Relapse after allogeneic hematopoietic cell therapy. Biol. Blood Marrow Transplant. 16, S138–S145 (2010).
Spyridonidis, A. et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia 26, 1211–1217 (2012).
Roddie, C. & Peggs, K. S. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin. Biol. Ther. 11, 473–487 (2011).
Kolb, H. J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 112, 4371–4383 (2008).
Frey, N. V. & Porter, D. L. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract. Res. Clin. Haematol. 21, 205–222 (2008).
Kochenderfer, J. N. et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122, 4129–4139 (2013).
Cruz, C. R. Y. et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 122, 2956–2973 (2013).
Brudno, J. N. et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 34, 1112–1121 (2016).
Ghosh, A. et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat. Med. 23, 242–249 (2017).
Qasim, W. et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9, eaaj2013 (2017).
Swerdlow, S. H. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissue (International Agency for Research on Cancer, 2008).
Till, B. G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Wang, Y. et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin. Immunol. 155, 160–175 (2014).
Zhang, W. Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduct. Target. Ther. 1, 16002 (2016).
Wang, C. et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin's lymphoma: an open-label phase I trial. Clin. Cancer Res. 23, 1156–1166 (2017).
Hiraga, J. et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood 113, 4885–4893 (2009).
Kennedy, G. A. et al. Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin's lymphoma: a retrospective review. Br. J. Haematol. 119, 412–416 (2002).
Haso, W. et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013).
Long, A. H., Haso, W. M. & Orentas, R. J. Lessons learned from a highly-active CD22-specific chimeric antigen receptor. Oncoimmunology 2, e23621 (2013).
Durkop, H. et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell 68, 421–427 (1992).
Stein, H. et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed–Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 66, 848–858 (1985).
Younes, A. et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 363, 1812–1821 (2010).
Younes, A. et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 14, 1348–1356 (2013).
Moskowitz, A. J. et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 16, 284–292 (2015).
Moskowitz, C. H. et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385, 1853–1862 (2015).
Pro, B. et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J. Clin. Oncol. 30, 2190–2196 (2012).
Horwitz, S. M. et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 123, 3095–3100 (2014).
Fanale, M. A. et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J. Clin. Oncol. 32, 3137–3143 (2014).
Deng, C., Pan, B. & O'Connor, O. A. Brentuximab vedotin. Clin. Cancer Res. 19, 22–27 (2013).
Hombach, A. et al. An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30. Cancer Res. 58, 1116–1119 (1998).
Hombach, A. et al. Characterization of a chimeric T-cell receptor with specificity for the Hodgkin's lymphoma-associated CD30 antigen. J. Immunother. 22, 473–480 (1999).
Savoldo, B. et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30ζ artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood 110, 2620–2630 (2007).
Di Stasi, A. et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113, 6392–6402 (2009).
Ramos, C. A., Heslop, H. E. & Brenner, M. K. CAR-T cell therapy for lymphoma. Ann. Rev. Med. 67, 165–183 (2016).
Ramos, C. A. et al. Chimeric T cells for therapy of CD30+ Hodgkin and non-Hodgkin lymphomas. Blood 126, 185 (2015).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl Med. 3, 95ra73 (2011).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Brentjens, R. Y. R., Bernal, Y., Riviere, I. & Sadelain, M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: a case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 18, 666–668 (2010).
Harris, J. Kite reports cerebral edema death in ZUMA-1 CAR T-Cell Trial. Onc Live http://www.onclive.com/web-exclusives/kite-reports-cerebral-edema-death-in-zuma1-car-tcell-trial#sthash.1UENjTT7.dpufhttp://www.onclive.com/web-exclusives/kite-reports-cerebral-edema-death-in-zuma1-car-tcell-trial (2017).
Uckun, F. M. et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood 71, 13–29 (1988).
Brudno, J. et al. T. cells expressing a novel fully-human anti-CD19 chimeric antigen receptor induce remissions of advanced lymphoma in a first-in-humans clinical trial [abstract 999]. ASH https://ash.confex.com/ash/2016/webprogram/Paper97536.html (2016).
Budde, L. E. et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE 8, e82742 (2013).
Diaconu, I. et al. Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol. Ther. 25, 580–592 (2017).
Künkele, A. et al. Functional tuning of CARs reveals signaling threshold above which CD8+ CTL antitumor potency is attenuated due to cell Fas–FasL-dependent AICD. Cancer Immunol. Res. 3, 368–379 (2015).
Alabanza, L., Pegues, M., Geldres, C., Shi, V. & Kochenderfer, J. The impact of different hinge and transmembrane components on the function of a novel fully-human anti-CD19 chimeric antigen receptor. Mol. Ther. 24, S32–S33 (2016).
Gargett, T. et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol. Ther. 24, 1135–1149 (2016).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Kenderian, S. S., Porter, D. L. & Gill, S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the CART before the horse. Biol. Blood Marrow Transplant. 23, 235–246 (2017).
Sommermeyer, D. et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia http://dx.doi.org/10.1038/leu.2017.57 (2017).
Ruella, M. et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 126, 3814–3826 (2016).
Schneider, D. et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J. Immunother. Cancer 5, 42 (2017).
Osborn, M. J. et al. Evaluation of TCR gene editing achieved by TALENs, CRISPR/Cas9, and megaTAL nucleases. Mol. Ther. 24, 570–581 (2016).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Sabatino, M. et al. Generation of clinical-grade CD19-specific CAR-modified CD81 memory stem cells for the treatment of human B-cell malignancies. Blood 128, 519–528 (2016).
Ali, S. A. et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 128, 1688–1700 (2016).
Ruella, M. et al. The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T Cells (CART19) improves responses against mantle cell lymphoma. Clin. Cancer Res. 22, 2684–2696 (2016).
Armand, P. Immune checkpoint blockade in hematologic malignancies. Blood 125, 3393–3400 (2015).
Goodman, A., Patel, S. P. & Kurzrock, R. PD-1–PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat. Rev. Clin. Oncol. 14, 203–220 (2017).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Chong, E. A. et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129, 1039–1041 (2017).
Vaxman, I. et al. Secondary malignancies following high dose therapy and autologous hematopoietic cell transplantation-systematic review and meta-analysis. Bone Marrow Transplant. 50, 706–714 (2015).
Pedersen-Bjergaard, J., Andersen, M. K. & Christiansen, D. H. Therapy-related acute myeloid leukemia and myelodysplasia after high-dose chemotherapy and autologous stem cell transplantation. Blood 95, 3273–3279 (2000).
Kochenderfer, J. N. et al. Long-duration complete remissions of diffuse large b-cell lymphoma after anti-CD19 chimeric antigen receptor therapy. Mol. Ther. http://dx.doi.org/10.1016/j.ymthe.2017.07.004 (2017).
Author information
Authors and Affiliations
Contributions
Both authors contributed to all stages of the preparation of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
J.N.K. receives research funding from cooperative research and development agreements between the National Cancer Institute (NCI) and Kite Pharma, and between the NCI and Bluebird Bio. J.N.K. also has multiple patent applications related to chimeric antigen receptors (CARs). J.N.B. declares no competing interests.
Rights and permissions
About this article
Cite this article
Brudno, J., Kochenderfer, J. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 15, 31–46 (2018). https://doi.org/10.1038/nrclinonc.2017.128
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.128
This article is cited by
-
Efficacy of programmed cell death 1 inhibitor maintenance after chimeric antigen receptor T cells in patients with relapsed/refractory B-cell non-Hodgkin-lymphoma
Cellular Oncology (2024)
-
Integrative analyses reveal outcome-associated and targetable molecular partnerships between TP53, BRD4, TNFRSF10B, and CDKN1A in diffuse large B-cell lymphoma
Annals of Hematology (2024)
-
Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy
Molecular Cancer (2023)
-
A non-genetic engineering platform for rapidly generating and expanding cancer-specific armed T cells
Journal of Biomedical Science (2023)
-
Protection of cell therapeutics from antibody-mediated killing by CD64 overexpression
Nature Biotechnology (2023)