Key Points
-
The definition of locally advanced breast cancer (LABC) can be broadened to include large operable tumours, however, we commonly use this term to refer to inoperable cancers
-
The management of LABC constitutes an important clinical problem, particularly in developing countries and those without widely adapted awareness programmes
-
The optimal management of LABC requires a multidisciplinary approach and collaboration between medical, surgical and radiation oncologists
-
Few data exist on LABC systemic treatment; the majority of data are from studies including both large-operable and locally advanced inoperable tumours—posing many challenges in the management of LABC
-
Several new molecularly targeted agents are under clinical investigation aiming to improve the clinical outcome of patients with LABC
-
The negative results of the ALTTO trial after promising data from NeoALTTO advocate a reassessment of pathological complete response as a suitable surrogate marker for long-term outcome in breast cancer
Abstract
Locally advanced breast cancer (LABC) constitutes a heterogeneous entity that includes advanced-stage primary tumours, cancers with extensive nodal involvement and inflammatory breast carcinomas. Although the definition of LABC can be broadened to include some large operable breast tumours, we use this term to strictly refer to inoperable cancers that are included in the above-mentioned categories. The prognosis of such tumours is often unfavourable; despite aggressive treatment, many patients eventually develop distant metastases and die from the disease. Advances in systemic therapy, including radiation treatment, surgical techniques and the development of new targeted agents have significantly improved clinical outcomes for patients with this disease. Notwithstanding these advances, LABC remains an important clinical problem, particularly in developing countries and those without widely adapted breast cancer awareness programmes. The optimal management of LABC requires a multidisciplinary approach, a well-coordinated treatment schedule and close cooperation between medical, surgical and radiation oncologists. In this Review, we discuss the current state of the art and possible future treatment strategies for patients with LABC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
14 April 2015
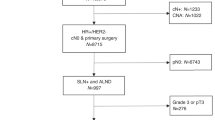
In the version of this article originally published online and in print, the word 'radiotherapy' was missing in figure 1 from one of the boxes. Additionally, several of the boxes had been merged, which meant the order of treatments was unclear. The figure has now been corrected for the HTML and PDF versions of the article published online.
References
Giordano, S. H. Update on locally advanced breast cancer. Oncologist 8, 521–530 (2003).
International Union Against Cancer. TNM classification of malignant tumours 7th edn (Wiley-Blackwell, 2009).
Taghian, A., El-Ghamry, M. & Merajver, S. Overview in the treatment of newly diagnosed, non- metastatic breast cancer. Uptodate [online], (2013).
NCCN. Management Guidelines for breast cancer [online], (2013).
Yamauchi, H. et al. Inflammatory breast cancer: what we know and what we need to learn. Oncologist 17, 891–899 (2012).
Kesson, E. M., Allardice, G. M., George, W. D., Burns, H. J. & Morrison, D. S. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ 344, e2718 (2012).
Jemal, A. et al. Cancer statistics. CA Cancer J. Clin. 59, 225–249 (2009).
Newman, L. A. Epidemiology of locally advanced breast cancer. Semin. Radiat. Oncol. 19, 195–203 (2009).
Allemani, C. et al. Breast cancer survival in the US and Europe: a CONCORD high-resolution study. Int. J. Cancer 132, 1170–1181 (2013).
Seidman, H., Gelb, S. K., Silverberg, E., LaVerda, N. & Lubera, J. A. Survival experience in the Breast Cancer Detection Demonstration Project. CA Cancer J. Clin. 37, 258–290 (1987).
American College of Surgeons. National Cancer Database [online], (2014).
El Saghir, N. S. et al. Breast cancer management in low resource countries (LRCs): consensus statement from the Breast Health Global Initiative. Breast 20 (Suppl. 2), S3–S11 (2011).
Berg, J. W. & Hutter, R. V. Breast cancer. Cancer 75, 257–269 (1995).
Chang, S., Parker, S. L., Pham, T., Buzdar, A. U. & Hursting, S. D. Inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results programme of the National Cancer Institute, 1975–1992. Cancer 82, 2366–2372 (1998).
Bonnefoi, H. et al. TP53 status for prediction of sensitivity to taxane versus non-taxane neoadjuvant chemotherapy in breast cancer (EORTC 10994/BIG 1–00): a randomised phase 3 trial. Lancet Oncol. 12, 527–539 (2011).
Buzdar, A. U. et al. Combined modality treatment of stage III and inflammatory breast cancer. MD Anderson Cancer Center experience. Surg. Oncol. Clin. N. Am. 4, 715–734 (1995).
Dawood, S. et al. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer 117, 1819–1826 (2011).
Tai, P. et al. Short- and long-term cause-specific survival of patients with inflammatory breast cancer. BMC Cancer 5, 137 (2005).
Dawood, S. et al. Survival of women with inflammatory breast cancer: a large population-based studydagger. Ann. Oncol. 25, 1143–1151 (2014).
Dawood, S. et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann. Oncol. 22, 515–523 (2011).
Le, M. G. et al. Are risk factors for breast cancer similar in women with inflammatory breast cancer and in those with non-inflammatory breast cancer? Breast 15, 355–362 (2006).
Hance, K. W., Anderson, W. F., Devesa, S. S., Young, H. A. & Levine, P. H. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results programme at the National Cancer Institute. J. Natl Cancer Inst. 97, 966–975 (2005).
Diab, S. G., Elledge, R. M. & Clark, G. M. Tumour characteristics and clinical outcome of elderly women with breast cancer. J. Natl Cancer Inst. 92, 550–556 (2000).
Yancik, R., Ries, L. G. & Yates, J. W. Breast cancer in aging women. A population-based study of contrasts in stage, surgery, and survival. Cancer 63, 976–981 (1989).
Bonnefoi, H. et al. Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: are there molecular markers in the primary tumour that predict for 5-year clinical outcome? Ann. Oncol. 14, 406–413 (2003).
Kleer, C. G., van Golen, K. L. & Merajver, S. D. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2, 423–429 (2000).
Wu, M. & Merajver, S. D. Molecular biology of inflammatory breast cancer: applications to diagnosis, prognosis, and therapy. Breast Dis. 22, 25–34 (2005).
Nguyen, D. M. et al. Molecular heterogeneity of inflammatory breast cancer: a hyperproliferative phenotype. Clin. Cancer Res. 12, 5047–5054 (2006).
Van der Auwera, I. et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin. Cancer Res. 10, 7965–7971 (2004).
Shirakawa, K. et al. Tumour-infiltrating endothelial cells and endothelial precursor cells in inflammatory breast cancer. Int. J. Cancer 99, 344–351 (2002).
Skobe, M. et al. Induction of tumour lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7, 192–198 (2001).
Stacker, S. A. et al. VEGF-D promotes the metastatic spread of tumour cells via the lymphatics. Nat. Med. 7, 186–191 (2001).
Kurebayashi, J. et al. Expression of vascular endothelial growth factor (VEGF) family members in breast cancer. Jpn J. Cancer Res. 90, 977–981 (1999).
Robbins, G. F., Shah, J., Rosen, P., Chu, F. & Taylor, J. Inflammatory carcinoma of the breast. Surg. Clin. N. Am. 54, 801–810 (1974).
van Golen, C. M. & van Golen, K. L. Inflammatory breast cancer stem cells: contributors to aggressiveness, metastatic spread and dormancy. J. Mol. Biomarkers Diagn. http://dx.doi.org/10.4172/2155-9929.S8-002 (2012).
van Golen, K. L., Wu, Z. F., Qiao, X. T., Bao, L. W. & Merajver, S. D. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 60, 5832–5838 (2000).
Kleer, C. G. et al. WISP3 is a novel tumour suppressor gene of inflammatory breast cancer. Oncogene 21, 3172–3180 (2002).
Bertucci, F. et al. Gene expression profiles of inflammatory breast cancer: correlation with response to neoadjuvant chemotherapy and metastasis-free survival. Ann. Oncol. 25, 358–365 (2014).
Van, L. S. et al. Distinct molecular signature of inflammatory breast cancer by cDNA microarray analysis. Breast Cancer Res. Treat. 93, 237–246 (2005).
Van Laere, S. J. et al. Uncovering the molecular secrets of inflammatory breast cancer biology: an integrated analysis of three distinct affymetrix gene expression datasets. Clin. Cancer Res. 19, 4685–4693 (2013).
Kleer, C. G., van Golen, K. L., Braun, T. & Merajver, S. D. Persistent E-cadherin expression in inflammatory breast cancer. Mod. Pathol. 14, 458–464 (2001).
Colpaert, C. G. et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br. J. Cancer 88, 718–725 (2003).
Qureshi, H. S., Linden, M. D., Divine, G. & Raju, U. B. E-cadherin status in breast cancer correlates with histologic type but does not correlate with established prognostic parameters. Am. J. Clin. Pathol. 125, 377–385 (2006).
Moll, U. M., Riou, G. & Levine, A. J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc. Natl Acad. Sci. USA 89, 7262–7266 (1992).
Davidoff, A. M., Humphrey, P. A., Iglehart, J. D. & Marks, J. R. Genetic basis for p53 overexpression in human breast cancer. Proc. Natl Acad. Sci. USA 88, 5006–5010 (1991).
Gonzalez-Angulo, A. M. et al. p53 expression as a prognostic marker in inflammatory breast cancer. Clin. Cancer Res. 10, 6215–6221 (2004).
Senkus, E. et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24 (Suppl. 6), vi7–vi23 (2013).
Symmans, W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–4422 (2007).
Nahleh, Z., Sivasubramaniam, D., Dhaliwal, S., Sundarajan, V. & Komrokji, R. Residual cancer burden in locally advanced breast cancer: a superior tool. Curr. Oncol. 15, 271–278 (2008).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804 (2012).
de Lena, M., Zucali, R., Viganotti, G., Valagussa, P. & Bonadonna, G. Combined chemotherapy-radiotherapy approach in locally advanced (T3b-T4) breast cancer. Cancer Chemother. Pharmacol. 1, 53–59 (1978).
Swain, S. M. et al. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res. 47, 3889–3894 (1987).
Hortobagyi, G. N. et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer 51, 763–768 (1983).
Powles, T. J. et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J. Clin. Oncol. 13, 547–552 (1995).
Fisher, B. et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 16, 2672–2685 (1998).
Semiglazov, V. F. et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann. Oncol. 5, 591–595 (1994).
Rueth, N. M. et al. Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: an analysis of treatment and survival trends from the National Cancer Database. J. Clin. Oncol. 32, 2018–2024 (2014).
Cardoso, F. 2nd. International concensus guidelines for advanced breast cancer (ABC2). Ann. Oncol. 25, 1871–1888 (2014); Breast 23, 489–502 (2014).
Heys, S. D. et al. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin. Breast Cancer 3 (Suppl. 2), S69–S74 (2002).
Smith, I. C. et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J. Clin. Oncol. 20, 1456–1466 (2002).
Eiermann, W. et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J. Clin. Oncol. 29, 3877–3884 (2011).
Earl, H. M. et al. Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high-risk early breast cancer (Neo-tAnGo): an open-label, 2x2 factorial randomised phase 3 trial. Lancet Oncol. 15, 201–212 (2014).
von Minckwitz, G. et al. Response-guided neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 31, 3623–3630 (2013).
von Minckwitz, G. et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J. Natl Cancer Inst. 100, 552–562 (2008).
Costa, S. D. et al. Neoadjuvant chemotherapy shows similar response in patients with inflammatory or locally advanced breast cancer when compared with operable breast cancer: a secondary analysis of the GeparTrio trial data. J. Clin. Oncol. 28, 83–91 (2010).
Byrski, T. et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 115, 359–363 (2009).
Sikov, W. M., Berry, D. A. & Perou C. M. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant weekly paclitaxel followeed by dose-dense AC on pathologic complete response in triple-negative breast cancer (TNBC): CALGB 40603 [abstract]. San Antonio Breast Cancer Symposium S5-01 (2013).
Sikov, W. M. et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 33, 13–21 (2014).
von Minckwitz, G., Schneeweiss, A. & Salata, C. A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple- negative and HER2 positive early breast cancer (GeparSixto). J. Clin. Oncol. 15, 747–756 (2014).
Petrelli, F. et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res. Treat. 144, 223–232 (2014).
von Minckwitz, G., Hahnen, E. & Fasching, P. Pathological complete response (p CR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (gBRCA) mutation and triple- negative breast cancer (TNBC):Results from GeparSixto [abstract]. J. Clin. Oncol. 32 (Suppl.), 1005 (2014).
Limentani, S. A., Brufsky, A. M., Erban, J. K., Jahanzeb, M. & Lewis, D. Phase II study of neoadjuvant docetaxel, vinorelbine, and trastuzumab followed by surgery and adjuvant doxorubicin plus cyclophosphamide in women with human epidermal growth factor receptor 2-overexpressing locally advanced breast cancer. J. Clin. Oncol. 25, 1232–1238 (2007).
Horiguchi, J. et al. Neoadjuvant weekly paclitaxel with and without trastuzumab in locally advanced or metastatic breast cancer. Anticancer Res. 29, 517–524 (2009).
Gianni, L. et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375, 377–384 (2010).
Untch, M. et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J. Clin. Oncol. 28, 2024–2031 (2010).
Untch, M. et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favourable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J. Clin. Oncol. 29, 3351–3357 (2011).
Baselga, J. et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379, 633–640 (2012).
Robidoux, A. et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 14, 1183–1192 (2013).
Hurvitz, S., Miller, J. & Dichman, R. Final analysis of a phase II, 3-arm, randomized trial of neoadjuvant trastuzumab or lapatinib or the combination of trastuzumab and lapatinib, followed by 6 cycles of docetaxel and carboplatin with trastuzumab and/or lapatinib in patients with HER2+ breast cancer (TRIO-US B07) [abstract]. San Antonio Breast Cancer Symposium S1-02 (2013).
Untch, M. et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 13, 135–144 (2012).
Piccart, M., Holmes, A. P. & Baselga, J. First results from the phase III ALTTO trial (BIG 2–06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L) [abstract]. J. Clin. Oncol. 32 (Suppl.), LBA4 (2014).
Guarneri, V. et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J. Clin. Oncol. 30, 1989–1995 (2012).
Piccart, M. J., Holmes, A. P. & de Azambuja, E. The association between event- free survival and pathological complete response to neoadjuvant lapatinib, trastuzumab or their combination in HER2 positive breast cancer. Survival follow-up analysis of the Neo ALTTO study (BIG 1–06) [abstract]. San Antonio Breast Cancer Symposium S1–01 (2013).
Carey, L., Berry, D. & Ollila, D. Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J. Clin. Oncol. 31 (Suppl.), 500 (2013).
Zardavas, D., Bozovic-Spasojevic, I. & de Azambuja, E. Dual human epidermal growth factor receptor 2 blockade: another step forward in treating patients with human epidermal growth factor receptor 2-positive breast cancer. Curr. Opin. Oncol. 24, 612–622 (2012).
Rimawi, M. F. et al. Multicentre phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J. Clin. Oncol. 31, 1726–1731 (2013).
Kuerer, H. M. et al. Clinical course of breast cancer patients with complete pathologic primary tumour and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J. Clin. Oncol. 17, 460–469 (1999).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24, 2278–2284 (2013).
Bear, H. D. et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N. Engl. J. Med. 366, 310–320 (2012).
von Minckwitz, G. et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med. 366, 299–309 (2012).
Earl, H., Hiller, L. & Blenkinshop C. ARTemis: a randomized trial of bevacizumab with neo-adjuvant chemotherapy (NACT) for patients with HER2 negative early breast cancer- primary endpoint, pathological complete response (p CR) [abstract]. J. Clin. Oncol. 32 (Suppl.), 1014 (2014).
Cardoso, F. et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast 21, 242–252 (2012).
Cristiciello, C., Alkalay, M. & Fumagali, L. Identification of a specific gene signature predictive of response to bevacizumab in patients with triple- negative inflammatory breast cancer [abstract]. Ann. Oncol. 24 (Suppl. 3), 30P (2013).
Wedam, S. B. et al. Anti-angiogenic and antitumour effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J. Clin. Oncol. 24, 769–777 (2006).
Overmoyer, B. et al. Inflammatory breast cancer as a model disease to study tumour angiogenesis: results of a phase IB trial of combination SU5416 and doxorubicin. Clin. Cancer Res. 13, 5862–5868 (2007).
Pierga, J. Y. et al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol. 13, 375–384 (2012).
Yardley, D. A. et al. Phase II study of neoadjuvant weekly nab-paclitaxel and carboplatin, with bevacizumab and trastuzumab, as treatment for women with locally advanced HER2+ breast cancer. Clin. Breast Cancer 11, 297–305 (2011).
Coudert, B., Pierga, J.-Y. & Mouret-Reynier, M.-A. AVATAXHER: A n open- label, randomized, multicentre study investigating the addition of bevacizumab (B) to neoadjuvant trastuzumab (T) plus docetaxel (D) in patients with early stage HER2 positive breast cancer (HER2+ BC) stratified according to PET change after one therapy cycle [abstract]. J. Clin. Oncol. 32 (Suppl.), 507 (2014).
Bozovic-Spasojevic, I. et al. Neoadjuvant anthracycline and trastuzumab for breast cancer: is concurrent treatment safe? Lancet Oncol. 12, 209–211 (2011).
Buzdar, A. U., Suman, V. & Bernstam F. ACOSOG Z1041 (Alliance): Definitive analysis of randomized neoadjuvant trial comparing FEC followed by paclitaxel plus trastuzumab with paclitaxel plus trastuzumab followed by FEC plus trastuzumab in HER2+ operable breast cancer. Lancet Oncol. 14, 1317 (2013).
Ewer, M., Suman, V. & Buzdar, A. ACOSOG Z1041 (Alliance): cardiac events (CE) among those receiving neoadjuvant antracyclines (A) and taxanes with trastuzumab (T) for HER2+ breast cancer[abstract]. J. Clin. Oncol. 31 (Suppl.), 526 (2013).
Ellis, M. J. et al. Outcome prediction for oestrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumour characteristics. J. Natl Cancer Inst. 100, 1380–1388 (2008).
Semiglazov, V. F. et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with oestrogen receptor-positive breast cancer. Cancer 110, 244–254 (2007).
Alba, E. et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006–03, a multicentre, randomized, phase-II study. Ann. Oncol. 23, 3069–3074 (2012).
Masuda, N. et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 13, 345–352 (2012).
Torrisi, R. et al. Letrozole plus GnRH analogue as preoperative and adjuvant therapy in premenopausal women with ER positive locally advanced breast cancer. Breast Cancer Res. Treat. 126, 431–441 (2011).
Ellis, M. J. et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with oestrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J. Clin. Oncol. 29, 2342–2349 (2011).
Krainick-Strobel, U. E. et al. Neoadjuvant letrozole in postmenopausal oestrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer 8, 62 (2008).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. 24, 2206–2223 (2013).
Touboul, E. et al. Possibility of conservative local treatment after combined chemotherapy and preoperative irradiation for locally advanced noninflammatory breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 34, 1019–1028 (1996).
Rastogi, P. et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 26, 778–785 (2008).
Thompson, A. M. & Moulder-Thompson, S. L. Neoadjuvant treatment of breast cancer. Ann. Oncol. 23 (Suppl. 10), x231–x236 (2012).
Whitman, G. J. & Strom, E. A. Workup and staging of locally advanced breast cancer. Semin. Radiat. Oncol. 19, 211–221 (2009).
Bogusevicius, A., Cepuliene, D. & Sepetauskiene, E. The integrated evaluation of the results of oncoplastic surgery for locally advanced breast cancer. Breast J. 20, 53–60 (2014).
Zucca Matthes, A. G. et al. Feasibility of oncoplastic techniques in the surgical management of locally advanced breast cancer. Int. J. Surg. 10, 500–505 (2012).
Lim, W. et al. Oncological safety of skin sparing mastectomy followed by immediate reconstruction for locally advanced breast cancer. J. Surg. Oncol. 102, 39–42 (2010).
Kuehn, T. et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 14, 609–618 (2013).
Boughey, J. C. et al. The role of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0-T4, N1–2) who receive neoadjuvant chemotherapy-Results from the ACOSOG Z1071 trial [abstract]. Cancer Res. 72, S2-1 (2012).
Kell, M. R. & Morrow, M. Surgical aspects of inflammatory breast cancer. Breast Dis. 22, 67–73 (2005).
Perez, C. A. et al. Management of locally advanced carcinoma of the breast. II. Inflammatory carcinoma. Cancer 74, 466–476 (1994).
Panades, M. et al. Evolving treatment strategies for inflammatory breast cancer: a population-based survival analysis. J. Clin. Oncol. 23, 1941–1950 (2005).
Newman, L. A. et al. Feasibility of immediate breast reconstruction for locally advanced breast cancer. Ann. Surg. Oncol. 6, 671–675 (1999).
Slavin, S. A., Love, S. M. & Goldwyn, R. M. Recurrent breast cancer following immediate reconstruction with myocutaneous flaps. Plast. Reconstr. Surg. 93, 1191–1204 (1994).
Olson, J. E. et al. The role of radiotherapy in the management of operable locally advanced breast carcinoma: results of a randomized trial by the Eastern Cooperative Oncology Group. Cancer 79, 1138–1149 (1997).
Overgaard, M. et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N. Engl. J. Med. 337, 949–955 (1997).
Overgaard, M. et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353, 1641–1648 (1999).
Recht, A. et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 17, 1689–1700 (1999).
Taghian, A. et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J. Clin. Oncol. 22, 4247–4254 (2004).
Katz, A. et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J. Clin. Oncol. 18, 2817–2827 (2000).
Toonkel, L. M., Fix, I., Jacobson, L. H. & Wallach, C. B. The significance of local recurrence of carcinoma of the breast. Int. J. Radiat. Oncol. Biol. Phys. 9, 33–39 (1983).
Ragaz, J. et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N. Engl. J. Med. 337, 956–962 (1997).
Ragaz, J. et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J. Natl Cancer Inst. 97, 116–126 (2005).
Huang, E. H. et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J. Clin. Oncol. 22, 4691–4699 (2004).
Mamounas, E. P. et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J. Clin. Oncol. 30, 3960–3966 (2012).
Kaufmann, M., Morrow, M., von Minckwitz, G. & Harris, J. R. Locoregional treatment of primary breast cancer: consensus recommendations from an International Expert Panel. Cancer 116, 1184–1191 (2010).
McGale, P. et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8,135 women in 22 randomised trials. Lancet 383, 2127–2135 (2014).
Bentzen, S. M. et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 9, 331–341 (2008).
Bentzen, S. M. et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 371, 1098–1107 (2008).
Whelan, T. J. et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 362, 513–520 (2010).
Bartelink, H. et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N. Engl. J. Med. 345, 1378–1387 (2001).
Budach, W., Kammers, K., Boelke, E. & Matuschek, C. Adjuvant radiotherapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials. Radiat. Oncol. 8, 267 (2013).
Calitchi, E. et al. Long-term results of neoadjuvant radiation therapy for breast cancer. Int. J. Cancer 96, 253–259 (2001).
Vernon, C. C. et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J. Radiat. Oncol. Biol. Phys. 35, 731–744 (1996).
Wust, P. et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 3, 487–497 (2002).
Zagar, T. M. et al. Durable palliation of breast cancer chest wall recurrence with radiation therapy, hyperthermia, and chemotherapy. Radiother Oncol. 97, 535–540 (2010).
Jones, E. L. et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin. Cancer Res. 10, 4287–4293 (2004).
Zardavas, D., Baselga, J. & Piccart, M. Emerging targeted agents in metastatic breast cancer. Nat. Rev. Clin. Oncol. 10, 191–210 (2013).
Simoncini, T. et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407, 538–541 (2000).
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 366, 520–529 (2012).
Piccart, M. et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann. Oncol. 25, 2357–2362 (2014).
Baselga, J. et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with oestrogen receptor-positive breast cancer. J. Clin. Oncol. 27, 2630–2637 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Zardavas, D., Fumagalli, D. & Loi, S. Phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway inhibition: a breakthrough in the management of luminal (ER+/HER2-) breast cancers? Curr. Opin. Oncol. 24, 623–634 (2012).
Zardavas, D., Phillips, W. A. & Loi, S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res. 16, 201 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Caldon, C. E. et al. Cyclin E2 overexpression is associated with endocrine resistance but not insensitivity to CDK2 inhibition in human breast cancer cells. Mol. Cancer Ther. 11, 1488–1499 (2012).
Finn, R. E. A. Final results of a randomized Phase II study of PD 0332991, a cyclin-dependent kinase (CDK)-4/6 inhibitor, in combination with letrozole vs letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1; TRIO-18) [abstract]. Cancer Res. 74, CT101 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Tu, Y., Hershman, D. & Pellegrino, C. Phase I–II study of the histone deacetylase inhibitor vorinostat plus sequential weekly paclitaxel and doxorubicin- cyclophosphamide in locally advanced breast cancer. Breast Cancer Res. Treat. 146, 145–152 (2014).
Rugo, H. S., Olopade, O. & De Michele, A. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: First efficacy results from the I-SPY 2 TRIAL [abstract]. San Antonio Breast Cancer Symposium, S5-02 (2013).
Gianni, L. et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J. Clin. Oncol. 23, 7265–7277 (2005).
Beitsch, P., Stork, L. & Snoo, F. Biomarker panel (TheraPrint) analysed as a predictor of response to neoadjuvant chemotherapy in patients with locally advanced breast cancer [abstract]. J. Clin. Oncol. 32 (Suppl.), 1026 (2014).
Fernandez, S. V. et al. Inflammatory breast cancer (IBC): clues for targeted therapies. Breast Cancer Res. Treat. 140, 23–33 (2013).
Tuma, R. S. ALK gene amplified in most inflammatory breast cancers. J. Natl Cancer Inst. 104, 87–88 (2012).
Gonzalez-Angulo, A. M., Hennessy, B. T. & Mills, G. B. Future of personalized medicine in oncology: a systems biology approach. J. Clin. Oncol. 28, 2777–2783 (2010).
Burock, S., Meunier, F. & Lacombe, D. How can innovative forms of clinical research contribute to deliver affordable cancer care in an evolving health care environment? Eur. J. Cancer 49, 2777–2783 (2013).
Gampenrieder, S. P., Rinnerthaler, G. & Greil, R. Neoadjuvant chemotherapy and targeted therapy in breast cancer: past, present, and future. J. Oncol. 2013, 732047 (2013).
Prowell, T. M. & Pazdur, R. Pathological complete response and accelerated drug approval in early breast cancer. N. Engl. J. Med. 366, 2438–2441 (2012).
Mohsin, S. K. et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J. Clin. Oncol. 23, 2460–2468 (2005).
Buzdar, A. U. et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin. Cancer Res. 13, 228–233 (2007).
Coudert, B. P. et al. Multicentre phase II trial of neoadjuvant therapy with trastuzumab, docetaxel, and carboplatin for human epidermal growth factor receptor-2-overexpressing stage II or III breast cancer: results of the GETN(A)-1 trial. J. Clin. Oncol. 25, 2678–2684 (2007).
Cataliotti, L. et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative 'Arimidex' Compared to Tamoxifen (PROACT) trial. Cancer 106, 2095–2103 (2006).
Ellis, M. J. & Ma, C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res. Treat. 105 (Suppl. 1), 33–43 (2007).
Smith, I. E. et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicentre double-blind randomized trial. J. Clin. Oncol. 23, 5108–5116 (2005).
Ellis, M. J. et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with oestrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J. Clin. Oncol. 29, 2342–2349 (2011).
Acknowledgements
K.T. has received grants from FOCA (Fonds Cancer-Belgium) and the Hellenic Society of Medical Oncology (HeSMO).
Author information
Authors and Affiliations
Contributions
K.T., E.S., M.J.C. and F.C. researched data for article, reviewed and edited the manuscript before submission, provided substantial contribution to discussion of content and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.S. is an advisory board member for Roche, has received travel support from Novartis and belongs to the speakers' bureau for AstraZeneca, GSK and Roche. F.C. has received Consultant/honouraria and is an advisory board member for Astellas, Astra-Zeneca, Celgene, Daiichi-Sankyo, Eisai, Genentech/Roche, GE Oncology, GlaxoSmithKline, Merck-Sharp, Merus, Novartis, Pfizer, and Sanofi. K.T. and M.J.C. have no competing interests.
Rights and permissions
About this article
Cite this article
Tryfonidis, K., Senkus, E., Cardoso, M. et al. Management of locally advanced breast cancer—perspectives and future directions. Nat Rev Clin Oncol 12, 147–162 (2015). https://doi.org/10.1038/nrclinonc.2015.13
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.13
This article is cited by
-
Fungating and Ulcerating Breast Cancer: Wound Closure Algorithm, Complications, and Survival Trends
Indian Journal of Surgical Oncology (2023)
-
Clonal evolution in primary breast cancers under sequential epirubicin and docetaxel monotherapy
Genome Medicine (2022)
-
High AUF1 level in stromal fibroblasts promotes carcinogenesis and chemoresistance and predicts unfavorable prognosis among locally advanced breast cancer patients
Breast Cancer Research (2022)
-
Survival comparison between postoperative and preoperative radiotherapy for stage I–III non-inflammatory breast cancer
Scientific Reports (2022)
-
The effect of metformin when combined with neoadjuvant chemotherapy in breast cancer patients
Medical Oncology (2022)