Key Points
-
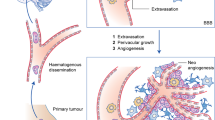
An increased understanding of the molecular biology of metastatic processes, including cell migration, blood–brain barrier penetration, angiogenesis and tumour proliferation, is providing new opportunities for the development of targeted therapies
-
Advances in MRI, incorporating spectroscopy and perfusion techniques, and tracers unique to metastases, provide additional information on responses to treatment and enable the earlier detection of new tumours
-
Improvements in intraoperative tumour identification using MRI and fluorescent agents maximize the likelihood of complete tumour resection and minimize injury to normal tissue
-
Reduction of radiation-induced cerebral injury and cognitive decline through repeated use of stereotactic radiosurgery or hippocampal-avoidance whole-brain radiotherapy provide useful options for individuals with advanced cerebral metastatic disease
-
Targeted therapy is beneficial in molecularly-selected tumours, including erlotinib in EGFR-mutant lung tumours, crizotinib in lung carcinomas with EML4–ALK translocations, trastuzumab in HER2+ breast cancer and dabrafenib in BRAF-mutant melanoma
Abstract
Metastatic tumours involving the brain overshadow primary brain neoplasms in frequency and are an important complication in the overall management of many cancers. Importantly, advances are being made in understanding the molecular biology underlying the initial development and eventual proliferation of brain metastases. Surgery and radiation remain the cornerstones of the therapy for symptomatic lesions; however, image-based guidance is improving surgical technique to maximize the preservation of normal tissue, while more sophisticated approaches to radiation therapy are being used to minimize the long-standing concerns over the toxicity of whole-brain radiation protocols used in the past. Furthermore, the burgeoning knowledge of tumour biology has facilitated the entry of systemically administered therapies into the clinic. Responses to these targeted interventions have ranged from substantial toxicity with no control of disease to periods of useful tumour control with no decrement in performance status of the treated individual. This experience enables recognition of the limits of targeted therapy, but has also informed methods to optimize this approach. This Review focuses on the clinically relevant molecular biology of brain metastases, and summarizes the current applications of these data to imaging, surgery, radiation therapy, cytotoxic chemotherapy and targeted therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
06 March 2014
In the version of this article initially published online, details of Jack Arbiser’s primary affiliation were omitted from the correspondence section, and should have included 'Atlanta Veterans Administration Medical Center'. The error has been corrected for the print, HTML and PDF versions of the article.
References
US Census Bureau. Census.gov [online], (2010).
Fox, B. D., Cheung, V. J., Patel, A. J., Suki, D. & Rao, G. Epidemiology of metastatic brain tumors. Neurosurg. Clin. N. Am. 22, 1–6 (2011).
Smedby, K. E., Brandt, L., Backlund, M. L. & Blomqvist, P. Brain metastases admissions in Sweden between 1987 and 2006. Br. J. Cancer 101, 1919–1924 (2009).
Nieder, C., Spanne, O., Mehta, M. P., Grosu, A. L. & Geinitz, H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer 117, 2505–2512 (2011).
Schouten, L. J., Rutten, J., Huveneers, H. A. & Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94, 2698–2705 (2002).
Barnholtz-Sloan, J. S. et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 22, 2865–2872 (2004).
Tabouret, E. et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 32, 4655–4662 (2012).
Posner, J. B. & Chernik, N. L. Intracranial metastases from systemic cancer. Adv. Neurol. 19, 579–592 (1978).
Percy, A. K. Neoplasms of the central nervous system: epidemiologic considerations. Neurology 20, 398–399 (1970).
Pickren, J. W., Lopez, G., Tsukada, Y. & Lane, W. W. Brain metastases: an autopsy study. Cancer Treat. Symp. 2, 295–313 (1983).
Tsukada, Y., Fouad, A., Pickren, J. W. & Lane, W. W. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 52, 2349–2354 (1983).
National Cancer institute. Seer.cancer.gov [online], (2013).
CDC. Decline in Breast Cancer Incidence—United States, 1999–2003. MMWR Morb. Mortal. Wkly Rep. 56, 549–553 (2007).
CDC Press release. Rates of new lung cancer cases drop in U. S. men and women [online], (2014).
Stemmler, H. J. et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 18, 23–38 (2007).
Pestalozzi, B. C., Brignoli, S. Trastuzumab in CSF. J. Clin. Oncol. 18, 2349–2351 (2000).
Wen, P. Y. & Loeffler, J. S. Management of brain metastases. Oncol. (Williston Park) 13, 941–954, 957–961; discussion 961–962, 9 (1999).
American Cancer Society. Cancer Facts & Figures 2013 [online], (2013).
Eichler, A. F. et al. The biology of brain metastases—translation to new therapies. Nat. Rev. Clin. Oncol. 8, 344–356 (2011).
Fidler, I. J., Balasubramanian, K., Lin, Q., Kim, S. W. & Kim, S. J. The brain microenvironment and cancer metastasis. Mol. Cells 30, 93–98 (2010).
Park, E. S. et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc. Natl Acad. Sci. USA 108, 17456–17461 (2011).
Kim, S. J. et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 13, 286–298 (2011).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Dvorak, H. F. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 20, 4368–4380 (2002).
Rampling, R. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 29, 427–431 (1994).
Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004).
Batchelor, T. T. et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 28, 2817–2823 (2010).
Kodack, D. P. et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc. Natl Acad. Sci. USA 109, E3119–E3127 (2012).
Pivot, X., Bedairia, N., Thiery-Vuillemin, A., Espie, M. & Marty, M. Combining molecular targeted therapies: clinical experience. Anticancer Drugs 22, 701–710 (2011).
Zhang, L. et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 5, 180ra48 (2013).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Sijens, P. E. et al. 1H MR spectroscopy in patients with metastatic brain tumors: a multicenter study. Magn. Reson. Med. 33 818–826 (2005).
Kelly, P. J. et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin. Proc. 62, 450–459 (1987).
Law, M. et al. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 222, 715–721 (2002).
Cha, S. Neuroimaging in neuro-oncology. Neurotherapeutics 6, 465–477 (2009).
Connell, J. J. et al. Selective permeablization of the blood–brain barrier at sites of metastasis. J. Natl Cancer Inst. 105, 1634–1643 (2013).
Serres, S. et al. Molecular MRI enables early and sensitive detection of brain metastases. Proc. Natl Acad. Sci. USA 109, 6674–6679 (2012).
Kalkanis, S. N. et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 96, 33–43 (2010).
Patchell, R. A. et al. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 322, 494–500 (1990).
Vecht, C. J. et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann. Neurol. 33, 583–590 (1993).
Katz, H. R. The relative effectiveness of radiation therapy, corticosteroids and surgery in the management of melanoma metastatic to the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 7, 897–906 (1981).
Bindal, R. K., Sawaya, R., Leavens, M. E. & Lee, J. J. Surgical treatment of multiple brain metastases. J. Neurosurg. 79, 210–216 (1993).
Bindal, R. K., Sawaya, R., Leavens, M. E., Hess, K. R. & Taylor, S. H. Reoperation for recurrent metastatic brain tumors. J. Neurosurg. 83, 600–604 (1995).
Al-Zabin, M., Ullrich, W. O., Brawanski, A. & Proescholdt, M. A. Recurrent brain metastases from lung cancer: the impact of reoperation. Acta Neurochir. (Wien) 152, 1887–1892 (2010).
Hatiboglu, M. A., Wildrick, D. M. & Sawaya, R. The role of surgical resection in patients with brain metastases. Ecancermedicalscience 7, 308 (2013).
Iwadate, Y., Namba, H. & Yamaura, A. Significance of surgical resection for the treatment of multiple brain metastases. Anticancer Res. 20, 573–577 (2000).
Andrews, D. W. et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363, 1665–1672 (2004).
Kondziolka, D., Patel, A., Lunsford, L. D., Kassam, A. & Flickinger, J. C. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 45, 427–434 (1999).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
McGirt, M. J. et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 65, 463–469 (2009).
Gulati, S. et al. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 76, 572–579 (2011).
Youmans, J. R. & Winn, R. H. (eds) Youmans Neurological Surgery. (Saunders/Elsevier, 2011).
Garber, S. T. & Jensen, R. L. Image guidance for brain metastases resection. Surg. Neurol. Int. 3, S111–S117 (2012).
Sills, A. K. Current treatment approaches to surgery for brain metastases. Neurosurgery 57, S24–S32 (2005).
Levitt, M. R., Levitt, R. & Silbergeld, D. L. Controversies in the management of brain metastases. Surg. Neurol. Int. 4, S231–S235 (2013).
Narang, J. et al. Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro. Oncol. 13, 1037–1046 (2011).
Eyüpoglu, I. Y., Buchfelder, M. & Savaskan, N. E. Surgical resection of malignant gliomas—role in optimizing patient outcome. Nat. Rev. Neurol. 9, 141–151 (2013).
Kellogg, R. G. & Munoz, L. F. Selective excision of cerebral metastases from the precentral gyrus. Surg. Neurol. Int. 4, 66 (2013).
Weil, R. J. & Lonser, R. R. Selective excision of metastatic brain tumors originating in the motor cortex with preservation of function. J. Clin. Oncol. 23, 1209–1217 (2005).
Walter, J., Kuhn, S. A., Waschke, A., Kalff, R. & Ewald, C. Operative treatment of subcortical metastatic tumours in the central region. J. Neurooncol. 103, 567–573 (2011).
Orringer, D. A., Golby, A. & Jolesz, F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev. Med. Devices 9, 491–500 (2012).
Schackert, G., Steinmetz, A., Meier, U. & Sobottka, S. B. Surgical management of single and multiple brain metastases: results of a retrospective study. Onkologie 24, 246–255 (2001).
Nimsky, C., Ganslandt, O., Tomandl, B., Buchfelder, M. & Fahlbusch, R. Low-field magnetic resonance imaging for intraoperative use in neurosurgery: a 5-year experience. Eur. Radiol. 12, 2690–2703 (2002).
Kubben, P. L. et al. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 12, 1062–1070 (2011).
Martirosyan, N. L. et al. Use of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor. J. Neurosurg. 115, 1131–1138 (2011).
Sanai, N. et al. Intraoperative confocal microscopy for brain tumors: a feasibility analysis in humans. Neurosurgery 68, 282–290 (2011).
Kircher, M. F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 18, 829–834 (2012).
Kalkanis, S. N. et al. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J. Neurooncol. http://dx.doi.org/10.1007/s11060-013-1326-9.
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Sahgal, A., Soliman, H. & Larson, D. A. Whole-brain radiation therapy of brain metastasis. Prog. Neurol. Surg. 25, 82–95 (2012).
Gaspar, L. et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 37, 745–751 (1997).
Kohler, B. A. et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J. Natl Cancer Inst. 103, 714–736 (2011).
Li, J. et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 71, 64–70 (2008).
DeAngelis, L. M., Delattre, J. Y. & Posner, J. B. Radiation-induced dementia in patients cured of brain metastases. Neurology 39, 789–796 (1989).
Welzel, G. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 73, 919–926 (2009).
Borgelt, B. et al. The palliation of brain metastases: final results of the first two studies by the radiation therapy oncology group. Int. J. Radiat. Oncol. Biol. Phys. 6, 1–9 (1980).
Murray, K. J. et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int. J. Radiat. Oncol. Biol. Phys. 39, 571–574 (1997).
Noordijk, E. M. et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int. J. Radiat. Oncol. Biol. Phys. 29, 711–717 (1994).
Addeo, R. et al. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer 113, 2524–2531 (2008).
Chua, D. et al. Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-small-cell lung cancer: a randomized, open-label phase II study. Clin. Lung Cancer 11, 176–181 (2010).
Kouvaris, J. R. et al. Phase II study of temozolomide and concomitant whole-brain radiotherapy in patients with brain metastases from solid tumors. Onkologie 30, 361–366 (2007).
Verger, E. et al. Temozolomide and concomitant whole-brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 61, 185–191 (2005).
Welsh, J. W. et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 31, 895–902 (2013).
Sperduto, P. W. et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int. J. Radiat. Oncol. Biol. Phys. 85, 1312–1318 (2013).
Aoyama, H. et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295, 2483–2491 (2006).
Sneed, P. K. et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int. J. Radiat. Oncol. Biol. Phys. 43, 549–558 (1999).
Sneed, P. K. et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 53, 519–526 (2002).
Chang, E. L. et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 10, 1037–1044 (2009).
Tsao, M., Xu, W. & Sahgal, A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 118, 2486–2493 (2012).
Aoyama, H. et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int. J. Radiat. Oncol. Biol. Phys. 68, 1388–1395 (2007).
Kocher, M. et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J. Clin. Oncol. 29, 134–141 (2011).
Soffietti, R. et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J. Clin. Oncol. 31, 65–72 (2013).
Sahgal, A. et al. Individual patient data meta-analysis of randomized controlled trials comparing stereotactic radiosurgery alone to SRS plus whole brain radiation therapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 87, 1187 (2013).
Barani, I. J., Larson, D. A. & Berger, M. S. Future directions in treatment of brain metastases. Surg. Neurol. Int. 4 (Suppl. 4), S220–S230 (2013).
Slotman, B. et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med. 357, 664–672 (2007).
Gore, E. M. et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of Radiation Therapy Oncology Group Study RTOG 0214. J. Clin. Oncol. 29, 272–278 (2011).
Sun, A. et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J. Clin. Oncol. 29, 279–286 (2011).
Brown, P. D. et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 15, 1429–1437 (2013).
Gondi, V. et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 78, 1244–1252 (2010).
Gutierrez, A. N. et al. Whole-brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int. J. Radiat. Oncol. Biol. Phys. 69, 589–597 (2007).
Gondi, V. et al. Memory preservation with conformal avoidance of the hippocampus during whole-brain radiotherapy (WBRT) for patients with brain metastases: primary endpoint results of RTOG 0933. Int. J. Radiat. Oncol. Biol. Phys. 87, 1186 (2013).
Hall, W. A., Djalilian, H. R., Nussbaum, E. S. & Cho, K. H. Long-term survival with metastatic cancer to the brain. Med. Oncol. 17, 279–286 (2000).
Maclean, J. et al. Multi-disciplinary management for patients with oligometastases to the brain: results of a 5 year cohort study. Radiat. Oncol. 8, 156 (2013).
Gril, B. et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin. Cancer Res. 17, 142–153 (2011).
Palmieri, D. et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 7, 1438–1445 (2009).
Shaw, A. T. et al. Clinical activity of the ALK inhibitor LDK378 in advanced, ALK-positive NSCLC [abstract]. J. Clin. Oncol. 31 (Suppl.), abstract 8010 (2013).
Fukuoka, M. et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 29, 2866–2874 (2011).
Sosman, J. A. et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 366, 707–714 (2012).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Kallioniemi, O. P. et al. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int. J. Cancer 49, 650–655 (1991).
Nie, F. et al. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer 118, 5198–5209 (2012).
Gril, B. et al. Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. Eur. J. Cancer 46, 1204–1210 (2010).
Rahmathulla, G., Toms, S. A. & Weil, R. J. The molecular biology of brain metastasis. J. Oncol. 2012, 723541 (2012).
Sun, M. et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin. Cancer Res. 15, 4829–4837 (2009).
Benedettini, E. et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am. J. Pathol. 177, 415–423 (2010).
Breindel, J. L. et al. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res. 73, 5053–5065 (2013).
Li, B. et al. Elevated PLGF contributes to small-cell lung cancer brain metastasis. Oncogene 32, 2952–2962 (2013).
Nguyen, D. X. et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138, 51–62 (2009).
Grinberg-Rashi, H. et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin. Cancer Res. 15, 1755–1761 (2009).
Wu, P. F. et al. Phosphorylated insulin-like growth factor-1 receptor (pIGF1R) is a poor prognostic factor in brain metastases from lung adenocarcinomas. J. Neurooncol. 115, 61–70 (2013).
Chen, G., Wang, Z., Liu, X. Y. & Liu, F. Y. High-level CXCR4 expression correlates with brain-specific metastasis of non-small cell lung cancer. World J. Surg. 35, 56–61 (2011).
Yoshimasu, T. et al. Increased expression of integrin alpha3beta1 in highly brain metastatic subclone of a human non-small cell lung cancer cell line. Cancer Sci. 95, 142–148 (2004).
Shintani, Y. et al. Overexpression of ADAM9 in non-small cell lung cancer correlates with brain metastasis. Cancer Res. 64, 4190–4196 (2004).
Minotti, V. et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer 20, 93–98 (1998).
Kelly, K. & Bunn, P. A. Jr. Is it time to reevaluate our approach to the treatment of brain metastases in patients with non-small cell lung cancer? Lung Cancer 20, 85–91 (1998).
Pietanza, M. C. et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin. Cancer Res. 18, 1138–1145 (2012).
Cortes, J. et al. Front-line paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology 64, 28–35 (2003).
Bernardo, G. et al. First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non-small-cell lung cancer: a phase II study. Cancer Invest. 20, 293–302 (2002).
Robinet, G. et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) Protocol 95–1. Ann. Oncol. 12, 59–67 (2001).
Heimberger, A. B. et al. Brain tumors in mice are susceptible to blockade of epidermal growth factor receptor (EGFR) with the oral, specific, EGFR-tyrosine kinase inhibitor ZD1839 (Iressa). Clin. Cancer Res. 8, 3496–3502 (2002).
Lai, C. S., Boshoff, C., Falzon, M. & Lee, S. M. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax 61, 91 (2006).
Yap, T. A. et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J. Clin. Oncol. 28, 3965–3972 (2010).
Kuiper, J. L. & Smit, E. F. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases—one with a remarkable thoracic response as well. Lung Cancer 80, 102–105 (2013).
Grommes, C. et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 13, 1364–1369 (2011).
Katayama, T. et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J. Thorac. Oncol. 4, 1415–1419 (2009).
Heon, S. et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin. Cancer Res. 18, 4406–4414 (2012).
Doebele, R. C. et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 118, 4502–4511 (2012).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013).
Camidge, D. R. et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 13, 1011–1019 (2012).
Camidge, D. R. et al. Updated results of a first-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies [abstract 3401]. Presented at the European Cancer Congress, 2013 (2013).
Solomon, B., Wilner, K. D. & Shaw, A. T. Current status of targeted therapy for anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Clin. Pharmacol. Ther. 95, 15–23 (2013).
Costa, D. B. et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J. Clin. Oncol. 20, e443–e445 (2011).
Tang, S. C. et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int. J. Cancer 134, 1484–1494 (2014).
Kinoshita, Y., Koga, Y., Sakamoto, A. & Hidaka, K. Long-lasting response to crizotinib in brain metastases due to EML4-ALK-rearranged non-small-cell lung cancer. BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2013-200867.
Kim, Y. H. et al. High-dose crizotinib for brain metastases refractory to standard-dose crizotinib. J. Thor. Oncol. 8, e85–e86 (2013).
Gandhi, L., Drappatz, J., Ramaiya, N. H. & Otterson, G. A. High-dose pemetrexed in combination with high-dose crizotinib for the treatment of refractory CNS metastases in ALK-rearranged non-small-cell lung cancer. J. Thorac. Oncol. 8, e3–e5 (2013).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869–10874 (2001).
Guiu, S. et al. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 Working Group Statement. Ann. Oncol. 23, 2997–3006 (2012).
Heitz, F. et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur. J. Cancer 45, 2792–2798 (2009).
Dawood, S. et al. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer 118, 4652–4659 (2012).
Fokstuen, T. et al. Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res. Treat. 62, 211–216 (2000).
Anders, C. K. et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer 117, 1602–1611 (2011).
Niwinska, A., Murawska, M. & Pogoda, K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann. Oncol. 21, 942–948 (2010).
Melisko, M. E., Moore, D. H., Sneed, P. K., De Franco, J. & Rugo, H. S. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J. Neurooncol. 88, 359–365 (2008).
Amir, E. et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J. Clin. Oncol. 30, 587–592 (2012).
Niikura, N. et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J. Clin. Oncol. 30, 593–599 (2012).
Sezgin, C., Gokmen, E., Esassolak, M., Ozdemir, N. & Goker, E. Risk factors for central nervous system metastasis in patients with metastatic breast cancer. Med. Oncol. 24, 155–161 (2007).
Ryberg, M. et al. Predictors of central nervous system metastasis in patients with metastatic breast cancer. A competing risk analysis of 579 patients treated with epirubicin-based chemotherapy. Breast Cancer Res. Treat. 91, 217–225 (2005).
Musolino, A. et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer 117, 1837–1846 (2011).
Pestalozzi, B. C. et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann. Oncol. 17, 935–944 (2006).
Ray, P. S. et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 70, 3870–3876 (2010).
Lorger, M., Krueger, J. S., O'Neal, M., Staflin, K. & Felding-Habermann, B. Activation of tumor cell integrin αvβ3 controls angiogenesis and metastatic growth in the brain. Proc. Natl Acad. Sci. USA 106, 10666–10671 (2009).
Nam, D. H. I. Activation of notch signaling in a xenograft model of brain metastasis. Clin. Cancer Res. 14, 4059–4066 (2008).
Zhang, S. et al. SRC family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res. 73, 5764–5774 (2013).
Saldana, S. M. et al. Inhibition of type I insulin-like growth factor receptor signaling attenuates the development of breast cancer brain metastasis. PLoS ONE 8, e73406 (2013).
Palmieri, D. et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin. Cancer Res. 15, 6148–6157 (2009).
Lee, Y. T. Breast carcinoma: pattern of metastasis at autopsy. J. Surg. Oncol. 23, 175–180 (1983).
Trudeau, M. E. et al. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG). Ann. Oncol. 17, 952–956 (2006).
Siena, S. et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann. Oncol. 21, 655–661 (2010).
Iwamoto, F. M. et al. A phase II trial of vinorelbine and intensive temozolomide for patients with recurrent or progressive brain metastases. J. Neurooncol. 87, 85–90 (2008).
Christodoulou, C. et al. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) phase II study. J. Neurooncol. 71, 61–65 (2005).
Ekenel, M., Hormigo, A. M., Peak, S., Deangelis, L. M. & Abrey, L. E. Capecitabine therapy of central nervous system metastases from breast cancer. J. Neurooncol. 85, 223–227 (2007).
Wang, M. L., Yung, W. K., Royce, M.E., Schomer, D. F. & Theriault, R. L. Capecitabine for 5-fluorouracil-resistant brain metastases from breast cancer. Am. J. Clin. Oncol. 24, 421–424 (2001).
Rivera, E. et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer 107, 1348–1354 (2006).
Lin, N. U. et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 26, 1993–1999 (2008).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009).
Metro, G. et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann. Oncol. 22, 625–630 (2011).
Sutherland, S. et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases—the UK experience. Br. J. Cancer 102, 995–1002 (2010).
Lin, N. U. et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J. Neurooncol. 105, 613–620 (2011).
Batchelor, T. et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 14, 64–71 (2013).
Bourdeanu, L., Reilly, A. A. & Luu, T. CNS metastases in breast cancer: a comparison report [abstract P6-11-12]. Presented at the San Antonio Breast Cancer Symposium 2013.
Fitzgerald, D. P. et al. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol. Cancer Ther. 11, 1959–1967 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Ro, J. et al. Clinical outcomes of HER2-positive metastatic breast cancer patients with brain metastasis treated with lapatinib and capecitabine: an open-label expanded access study in Korea. BMC Cancer 12, 322 (2012).
Yap, Y. S. et al. Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br. J. Cancer 107, 1075–1082 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 366, 520–529 (2012).
Curran, M. P. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr. Drugs 14, 51–60 (2012).
Bendell, J. C. et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 30, 282–290 (2012).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
O'Shaughnessy, J. et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 364, 205–214 (2011).
Mehta, M. P. et al. Phase I safety and pharmacokinetic (PK) study of veliparib in combination with whole brain radiation therapy (WBRT) in patients (pts) with brain metastases [abstract 2013]. Int. J. Radiat. Oncol. Biol. Phys. 84 (Suppl.), S269–S270 (2012).
Byrne, T. N., Cascino, T. L. & Posner, J. B. Brain metastasis from melanoma. J. Neurooncol. 1, 313–317 (1983).
Davies, M. A. et al. Integrated molecular and clinical analysis of Akt activation in metastatic melanoma. Clin. Cancer Res. 15, 7538–7546 (2009).
Govindarajan, B. et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Invest. 117, 719–729 (2007).
Bonner, M. Y. & Arbiser, J. L. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell. Mol. Life Sci. 69, 2435–2442 (2012).
Boivin, B., Zhang, S., Arbiser, J. L., Zhang, Z. Y. & Tonks, N. K. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc. Natl Acad. Sci. USA 105, 9959–9964 (2008).
Munson, J. M. et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl. Med. 4, 127–136 (2012).
Hamilton, R. et al. Pathologic and gene expression features of metastatic melanomas to the brain. Cancer 119, 2737–2746 (2013).
Arpaia, E. et al. The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and ERK. Oncogene 31, 884–896 (2012).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Arnold, S. M. et al. Expression of p53, bcl-2, E-cadherin, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 in paired primary tumors and brain metastasis. Clin. Cancer Res. 5, 4028–4033 (1999).
Kusters, B. et al. Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res. 63, 5408–5413 (2003).
Kusters, B. et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 62, 341–345 (2002).
Yano, S. et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 60, 4959–4967 (2000).
Xie, T. X. et al. Activation of STAT3 in human melanoma promotes brain metastasis. Cancer Res. 66, 3188–3196 (2006).
Shaw, E. et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int. J. Radiat. Oncol. Biol. Phys. 47, 291–298 (2000).
Margolin, K. et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 13, 459–465 (2012).
Kingsley, D. P. An interesting case of possible abscopal effect in malignant melanoma. Br. J. Radiol. 48, 863–866 (1975).
Stamell, E. F. et al. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 85, 293–295 (2013).
Falchook, G. S. et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379, 1893–1901 (2012).
Long, G. V. et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 1087–1095 (2012).
Jang, S. & Atkins, M. B. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 14, e60–e69 (2013).
Okwan-Duodu, D., Pollack, B. P., Lawson, D. & Khan, M. K. Role of radiation therapy as immune activator in the era of modern immunotherapy for metastatic malignant melanoma. Am. J. Clin. Oncol. http://dx.doi.org/10.1097/COC.0b013e3182940dc3.
Martinez, N., Boire, A. & Deangelis, L. M. Molecular interactions in the development of brain metastases. Int. J. Mol. Sci. 14, 17157–17167 (2013).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Ceresoli, G. L. et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann. Oncol. 15, 1042–1047 (2004).
Wu, C. et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 57, 359–364 (2007).
Kim, J. E. et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 65, 351–354 (2009).
Hotta, K. et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer 46, 255–261 (2004).
Porta, R. et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur. Respir. J. 37, 624–631 (2011).
Atkins, M. B. et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer 113, 2139–2145 (2008).
Konstantinou, M. P. et al. Ipilimumab in melanoma patients with brain metastasis: a retrospective multicentre evaluation of thirty-eight patients. Acta Derm. Venereol. 94, 45–49 (2014).
Author information
Authors and Affiliations
Contributions
T.K.O., J.A., A.Z., A.R. and M.K.M. researched the data for this article. T.K.O., J.A., A.Z., H.-K.G.S., A.M.R., S.N.K. and J.J.O. made substantial contributions to all other stages of the preparation of the manuscript for submission. In addition, T.R. and K.M.E. contributed substantially to discussion of content and the writing of the article. T.G.W., B.S., N.L.T. and M.K.M. also made considerable contributions to the writing of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Owonikoko, T., Arbiser, J., Zelnak, A. et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11, 203–222 (2014). https://doi.org/10.1038/nrclinonc.2014.25
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.25
This article is cited by
-
Radiosurgery of limited brain metastases from primary solid tumor: results of the randomized phase III trial (NCT02355613) comparing treatments executed with a specialized or a C-arm linac-based platform
Radiation Oncology (2023)
-
Construction and evaluation of a gated high-resolution neural network for automatic brain metastasis detection and segmentation
European Radiology (2023)
-
Pregabalin mitigates microglial activation and neuronal injury by inhibiting HMGB1 signaling pathway in radiation-induced brain injury
Journal of Neuroinflammation (2022)
-
Hypoxia-induced GLT8D1 promotes glioma stem cell maintenance by inhibiting CD133 degradation through N-linked glycosylation
Cell Death & Differentiation (2022)
-
Preoperative frailty measured by risk analysis index predicts complications and poor discharge outcomes after Brain Tumor Resection in a large multi-center analysis
Journal of Neuro-Oncology (2022)