Abstract
Breast cancer is a heterogeneous disease with different molecular drivers regulating its growth, survival and response to therapy. Breast cancer is divided in three major subtypes based on the pattern of expression of hormone receptors and HER2: luminal tumors (or HR positive), HER2 amplified tumors, and the remaining subtypes being collectively referred to as triple-negative breast cancer. While tumors within these subtypes have similar gene-expression patterns, clinical outcomes, and response to therapy, this division is far from perfect and subgroups within these groups are beginning to be identified. In terms of therapy, an increasingly rational drug development effort has resulted in agents against new molecular targets that are active against only those tumors with the targeted molecular alteration or phenotype. These agents include receptor and non-receptor tyrosine kinase inhibitors (HER1, HER2, HER3, insulin-like growth factor receptor, c-met, fibroblast growth factor receptor and HSP 90 inhibitors), intracellular signaling pathways (PI3K, AKT, mTOR), angiogenesis inhibitors and agents that interfere with DNA repair (PARP inhibitors). Thus, the overall management of breast cancer will increasingly require an understanding of breast cancer heterogeneicity, the biological nature of any given tumor as well the existence of increased personalized treatment options.
Key Points
-
Breast cancer is a heteregenous disease divided in three major subtypes: HR positive, HER2 amplified, and triple negative; the clinical outcomes and treatments are different for each subgroup
-
The estrogen receptor, receptor tyrosine kinase and DNA repair pathways are key to understanding the growth and progression of invasive breast cancer and how interference of these pathways results in anti-cancer activity
-
A high number of molecular targeted agents are under clinical development and combinatorial approaches will probably become the norm
-
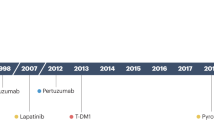
Targeted agents currently used in clinical practice are the anti-HER2 monoclonal antibody trastuzumab, the dual HER1/HER2 tyrosine kinase inhibitor lapatinib, and the anti-VEGF antibody bevacizumab
-
Studies with compounds targeting non-receptor tyrosine kinase (src), signaling pathways downstream of growth factor receptors (mTOR, PI3K, HSP90), and DNA repair mechanisms (PARP) have shown promising clinical activity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Perou, C. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Mauri, D., Pavlidis, N., Polyzos, N. P. & Ioannidis, J. P. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J. Natl Cancer Inst. 98, 1285–1291 (2006).
The Breast International Group 1–98 Collaborative Group et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N. Engl. J. Med. 353, 2747–2757 (2005).
Forbes, J. F. et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 9, 45–53 (2008).
Chia, S. et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J. Clin. Oncol. 26, 1664–1670 (2008).
Martin, L. A., Farmer, I., Johnston, S. R., Ali, S. & Dowsett, M. Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocr. Relat. Cancer 12 (Suppl. 1), S75–S84 (2005).
Bergh, J. et al. First results from FACT—An open-label, randomized phase III study investigating loading dose of fulvestrant combined with anastrozole versus anastrozole at first relapse in hormone receptor positive breast cancer [abstract]. Cancer Res. 69 (Suppl. 3), S490 a23 (2009).
Klijn, J. G. et al. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J. Clin. Oncol. 19, 343–353 (2001).
Rouzier, R. et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 11, 5678–5685 (2005).
Prat, A. & Baselga, J. The role of hormonal therapy in the management of hormonal-receptor-positive breast cancer with co-expression of HER2. Nat. Clin. Pract. Oncol. 5, 531–542 (2008).
De Laurentiis, M. et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin. Cancer Res. 11, 4741–4748 (2005).
Kaufman, B. et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone-receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J. Clin. Oncol. 27, 5529–5537 (2009).
Johnston, S. et al. Lapatinib combined with letrozole vs letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin. Oncol. 27, 5538–5546 (2009).
Baselga, J. et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J. Clin. Oncol. 23, 5323–5333 (2005).
Green, M. D. et al. Gefitinib treatment in hormone-resistant and hormone receptor-negative advanced breast cancer. Ann. Oncol. 20, 1813–1817 (2009).
Agrawal, A. et al. Efficacy and tolerability of gefitinib in oestrogen receptor negative and tamoxifen resistant oestrogen receptor positive locally advanced or metastatic breast cancer. Breast Cancer Res. Treat. 94 (Suppl. 1), S61 (2005).
Osborne, K. et al. Randomized phase II study of gefitinib (IRESSA) or placebo in combination with tamoxifen in patients with hormone receptor positive metastatic breast cancer. Breast Cancer Res. Treat. 106 (Suppl. 1), S107 (2007).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Stemke-Hale, K. et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 68, 6084–6091 (2008).
Serra, V. et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 68, 8022–8030 (2008).
Markman, B., Atzori, F., Perez-Garcia, J., Tabernero, J. & Baselga, J. Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann. Oncol. doi:10.1093/annonc/mdp347.
Baselga, J. et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 27, 2630–2637 (2009).
Chow, L. et al. Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer [abstract]. Breast Cancer Res. Treat. 100 (Suppl. 1), a6091 (2006).
Tabernero, J. et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J. Clin. Oncol. 26, 1603–1610 (2008).
Di Cosimo, S. et al. Combination of the mammalian target of rapamycin (mTOR) inhibitor everolimus (E) with the insulin like growth factor-1-receptor (IGF-1-R) inhibitor NVP-AEW-541: a mechanistic based anti-tumor strategy [abstract]. J. Clin. Oncol. 23, a3112 (2005).
Di Cosimo, S. et al. The PI3-K/AKT/mTOR pathway as a target for breast cancer therapy. J. Clin. Oncol. 25 (Suppl. 18), 3511 (2007).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
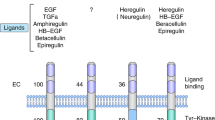
Baselga, J. & Swain, S. M. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 9, 463–475 (2009).
von Minckwitz, G. et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J. Clin. Oncol. 27, 1999–2006 (2009).
Franklin, M. et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5, 317–328 (2004).
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003).
Mendoza, N., Phillips, G. L., Silva, J., Schwall, R. & Wickramasinghe, D. Inhibition of ligand-mediated HER2 activation in androgen-independent prostate cancer. Cancer Res. 62, 5485–5488 (2002).
Agus, D. B. et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2, 127–137 (2002).
Portera, C. C. et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin. Cancer Res. 14, 2710–2716 (2008).
Baselga, J. et al. A phase II trial of pertuzumab and trastuzumab in patients with HER2-positive metastatic breast cancer that had progressed during prior trastuzumab therapy. J. Clin. Oncol. (in press).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Vogel, C. et al. Trastuzumab-DM1 (T-DM1), a HER2 antibody drug conjugate (ADC), in patients (pts) with HER2+ metastatic breast cancer (MBC): Final results [abstract]. J. Clin. Oncol. 27 (Suppl.), a1017 (2009).
Modi, S. et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J. Clin. Oncol. 25, 5410–5417 (2007).
Berns, K. et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12, 395–402 (2007).
Eichhorn, P. J. et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 68, 9221–9230 (2008).
Hurtitz, S. et al. Everolimus (RAD001) in combination with weekly paclitaxel and trastuzumab in patients (pts) with HER2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab: a multicenter phase I clinical trial [abstract]. Eur. J. Cancer 7 (Suppl.), a267 (2009).
Cardoso, F. et al. Multicenter phase I clinical trial of daily and weekly everolimus (RAD001) in combination with vinorelbine and trastuzumab in patients with HER-2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab [abstract]. Eur. J. Cancer 7 (Suppl.) O-5004 (2009).
Scaltriti, M. et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl Cancer Inst. 99, 628–638 (2007).
Lin, N., Dieras, V. & Paul, D. EGF105084, a phase II study of lapatinib for brain metastases in patients (pts) with HER2+ breast cancer following trastuzumab (H) based systemic therapy and cranial radiotherapy (RT) [abstract]. J. Clin. Oncol. 25 (Suppl.), a1012 (2007).
Burstein, H. et al. Neratinib (HKI-272), an irreversible pan erbB receptor tyrosine kinase inhibitor: phase 2 results in patients with advanced HER2+ breast cancer [abstract]. Cancer Res. 69, (Suppl.) a37 (2008).
Anders, C. & Carey, L. A. Understanding and treating triple-negative breast cancer. Oncology (Williston Park) 22, 1233–1240 (2008).
Sparano, J. A. et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 358, 1663–1671 (2008).
Carey, L. A. et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 13, 2329–2334 (2007).
Lee, F. Y. et al. Preclinical studies of ixabepilone (BMS-247550) demonstrate optimal antitumor activity against both chemotherapy-sensitive and resistant tumor types [abstract]. Cancer Res. 47, a503 (2006).
Thomas, E. S. et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J. Clin. Oncol. 25, 5210–5217 (2007).
Baselga, J. et al. Phase II genomics study of ixabepilone as neoadjuvant treatment for breast cancer. J. Clin. Oncol. 27, 526–534 (2009).
Carey, L. A. et al. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer [abstract]. J. Clin. Oncol. 26 (Suppl.), a1009 (2008).
O'Shaughnessy, J. et al. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer [abstract]. Breast Cancer Res. Treat. 106, S308 (2007).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Tutt, A. et al. Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer [abstract]. J. Clin. Oncol. 27 (Suppl. 18), a501 (2009).
Virág, L. & Szabó, C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 54, 375–429 (2002).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
O'Shaughnessy, J. et al. Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): Results of a randomized phase II trial. J. Clin. Oncol. 27 (Suppl. 18) a3 (2009).
Miller, K. D. et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 23, 792–799 (2005).
Miller, K. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 357, 2666–2676 (2007).
Miles, D. et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO [abstract]. J. Clin. Oncol. 26 (Suppl.), a1011 (2008).
Pàez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Ebos, J. M. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Robert, N. J. et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) [abstract]. J. Clin. Oncol. 27 (Suppl. 15), a1005 (2009).
Deprimo, S. et al. Effect of treatment with sunitinib malate, a multitargeted tyrosine kinase inhibitor, on circulating plasma levels of VEGF, soluble VEGF receptors 2 and 3, and soluble KIT in patients with metastatic breast cancer [abstract]. J. Clin. Oncol. 24, a578 (2006).
Miller, K. et al. Phase II study of SU11248, a multitargeted receptor tyrosine kinase inhibitor (TKI), in patients (pts) with previously treated metastatic breast cancer (MBC) [abstract]. J. Clin. Oncol. 23 (Suppl.), a563 (2005).
Wilhelm, S. M. et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Baselga, J. et al. SOLTI-0701: A double-blind, randomized phase 2b study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with capecitabine (CAP) in patients (pts) with locally advanced (adv) or metastatic (met) breast cancer (BC) [abstract]. Eur. J. Cancer 7 (Suppl.), 3LBA (2009).
Martin, G. S. The hunting of the Src. Nat. Rev. Mol. Cell Biol. 2, 467–475 (2001).
Thomas, S. M. & Brugge, J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 13, 513–609 (1997).
Yeatman, T. J. A renaissance for SRC. Nat. Rev. Cancer 4, 470–480 (2004).
Bromann, P. A., Korkaya, H. & Courtneidge, S. A. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23, 7957–7968 (2004).
Avizienyte, E. & Frame, M. C. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell. Biol. 17, 542–547 (2005).
Verbeek, B. S. et al. c-Src potein expression increased in human breast cancer. An immunohistochemical and biochemical analysis. J. Pathol. 180, 383–388 (1996).
Ottenhoff-Kalff, A. E. et al. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 52, 4773–4778 (1992).
Guy, C. T., Muthuswamy, S. K., Cardiff, R. D., Soriano, P. & Muller, W. J. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 8, 23–32 (1994).
Lombardo, L. J. et al. Discovery of N-(2-Chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 47, 6658–6661 (2004).
Hennequin, L. F. et al. N-(5-Chloro-1, 3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J. Med. Chem. 49, 6465–6488 (2006).
Tabernero, J. et al. Phase I study of AZD0530, an oral potent inhibitor of Src kinase: First demonstration of inhibition of Src activity in human cancers [abstract]. J. Clin. Oncol. 25 (Suppl.), a3520 (2007).
Elbauomy Elsheikh, S. et al. FGFR1 amplification in breast carcinomas: a chromogenic in situ hybridisation analysis. Breast Cancer Res. 9, R23 (2007).
Ponzo, M. G. et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc. Natl Acad. Sci. USA 106, 12903–12908 (2009).
Shattuck, D. L., Miller, J. K., Carraway, K. L. 3rd & Sweeney, C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 68, 1471–1477 (2008).
O'Shaughnessy, J. et al. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy [abstract]. J. Clin. Oncol. 26 (Suppl.), a1015 (2008).
Jerusalem, G. H. et al. Multicenter phase I clinical trial of daily and weekly RAD001 in combination with vinorelbine and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab [abstract]. J. Clin. Oncol. 26 (Suppl.), a1057 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J. Baselga declares he is a Consultant for Exelixis, Merck, Novartis and Roche. S. Di Cosimo declares no competing interests.
Rights and permissions
About this article
Cite this article
Di Cosimo, S., Baselga, J. Management of breast cancer with targeted agents: importance of heterogenicity. Nat Rev Clin Oncol 7, 139–147 (2010). https://doi.org/10.1038/nrclinonc.2009.234
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.234
This article is cited by
-
Quantitative proteomics reveals stage-specific protein regulation of triple negative breast cancer
Breast Cancer Research and Treatment (2021)
-
Inhibition of O-GlcNAc transferase activates tumor-suppressor gene expression in tamoxifen-resistant breast cancer cells
Scientific Reports (2020)
-
Metabolomics-Based Discovery of Molecular Signatures for Triple Negative Breast Cancer in Asian Female Population
Scientific Reports (2020)
-
ZMYND10, an epigenetically regulated tumor suppressor, exerts tumor-suppressive functions via miR145-5p/NEDD9 axis in breast cancer
Clinical Epigenetics (2019)
-
Improved contrast of affibody-mediated imaging of HER3 expression in mouse xenograft model through co-injection of a trivalent affibody for in vivo blocking of hepatic uptake
Scientific Reports (2019)