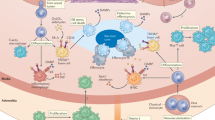
Key Points
-
Natural killer T (NKT) cells are activated by the interaction of CD1d molecules containing antigenic exogenous lipids or endogenous self-lipids on antigen-presenting cells, with the semi-invariant T cell receptor on NKT cells
-
Antigenic lipids include glycolipids and phospholipids of microbial origin or self-lipids
-
NKT cells can also be activated in a CD1d-independent manner by Toll-like receptor stimulation of IL-18 and IL-22 secretion
-
Activation of NKT cells results in the rapid release of TH1, TH2, and TH17 cytokines and the cytotoxic proteins perforin and granzyme B
-
Numerous murine studies have shown that NKT cells are proatherogenic; NKT cells can promote atherosclerosis by activation of their secreted cytokines on immune cells present in the atherosclerotic lesion and by the induction of apoptosis by cytotoxic proteins
Abstract
Atherosclerosis is a chronic inflammatory disorder that develops in response to hyperlipidaemia. Cells from both the innate and adaptive immune systems contribute to the development of atherosclerotic lesions. The role of natural killer T (NKT) cells in response to microbial pathogens and inflammatory disorders such as atherosclerosis has received increasing attention in the past 10–15 years. Endogenous self-lipid antigens and exogenous lipid antigens, including those on microorganisms can activate NKT cells. CD1d molecules on antigen-presenting cells present these lipids to the T-cell receptor on NKT cells, which results in the rapid production of cytokines and cytotoxic proteins. NKT cells can also be activated in a CD1d-independent manner. Numerous studies have shown that NKT cells can promote atherogenesis. Various NKT cell sublineages have been described, but the participation of each in atherogenesis requires further characterization. In this Review, we provide an overview of NKT cells in the immune system, discuss CD1 molecules and lipid antigen presentation, and describe the interaction of the CD1d–NKT cell network with gut microbiota and its effect in modulating the activity or levels of NKT cells, which might in turn influence atherosclerosis. Although the exact mechanisms by which NKT cells promote atherosclerosis have not been fully elucidated, several potential mechanisms are proposed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Witztum, J. L. & Lichtman, A. H. The influence of innate and adaptive immune responses on atherosclerosis. Annu. Rev. Pathol. 9, 73–102 (2014).
Hilgendorf, I., Swirski, F. K. & Robbins, C. S. Monocyte fate in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35, 272–279 (2015).
Nahrendorf, M. & Swirski, F. K. Abandoning M1/M2 for a network model of macrophage function. Circ. Res. 119, 414–417 (2016).
Bendelac, A., Killeen, N., Littman, D. R. & Schwartz, R. H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science 263, 1774–1778 (1994).
Liao, C. M., Zimmer, M. I. & Wang, C. R. The functions of type I and type II natural killer T cells in inflammatory bowel diseases. Inflamm. Bowel Dis. 19, 1330–1338 (2013).
Constantinides, M. G. & Bendelac, A. Transcriptional regulation of the NKT cell lineage. Curr. Opin. Immunol. 25, 161–167 (2013).
Brennan, P. J., Brigl, M. & Brenner, M. B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13, 101–117 (2013).
Bendelac, A., Savage, P. B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Van Kaer, L., Parekh, V. V. & Wu, L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 34, 50–58 (2013).
Van Kaer, L., Parekh, V. V. & Wu, L. The response of CD1d-restricted invariant NKT cells to microbial pathogens and their products. Front. Immunol. 6, 226 (2015).
Lee, Y. J., Holzapfel, K. L., Zhu, J., Jameson, S. C. & Hogquist, K. A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14, 1146–1154 (2013).
Monteiro, M. et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-beta. J. Immunol. 185, 2157–2163 (2010).
Sag, D., Krause, P., Hedrick, C. C., Kronenberg, M. & Wingender, G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J. Clin. Invest. 124, 3725–3740 (2014).
Lynch, L. et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat. Immunol. 16, 85–95 (2015).
Van Rhijn, I., Godfrey, D. I., Rossjohn, J. & Moody, D. B. Lipid and small-molecule display by CD1 and MR1. Nat. Rev. Immunol. 15, 643–654 (2015).
Zimmer, M. I. et al. Polymorphisms in CD1d affect antigen presentation and the activation of CD1d-restricted T cells. Proc. Natl Acad. Sci. USA 106, 1909–1914 (2009).
Vartabedian, V. F., Savage, P. B. & Teyton, L. The processing and presentation of lipids and glycolipids to the immune system. Immunol. Rev. 272, 109–119 (2016).
Anderson, B. L., Teyton, L., Bendelac, A. & Savage, P. B. Stimulation of natural killer T cells by glycolipids. Molecules 18, 15662–15688 (2013).
Zajonc, D. M. & Girardi, E. Recognition of microbial glycolipids by natural killer T cells. Front. Immunol. 6, 400 (2015).
Ly, D. & Moody, D. B. The CD1 size problem: lipid antigens, ligands, and scaffolds. Cell. Mol. Life Sci. 71, 3069–3079 (2014).
Felio, K. et al. CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J. Exp. Med. 206, 2497–2509 (2009).
Hansson, G. K. & Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 (2011).
Yanaba, K. et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28, 639–650 (2008).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Matsuda, J. L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000).
Barral, D. C. & Brenner, M. B. CD1 antigen presentation: how it works. Nat. Rev. Immunol. 7, 929–941 (2007).
Zeissig, S. et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J. Clin. Invest. 120, 2889–2899 (2010).
Tupin, E., Kinjo, Y. & Kronenberg, M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417 (2007).
Schrantz, N. et al. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J. Exp. Med. 204, 841–852 (2007).
Adams, E. J. Diverse antigen presentation by the group 1 CD1 molecule, CD1c. Mol. Immunol. 55, 182–185 (2013).
Kinjo, Y., Kitano, N. & Kronenberg, M. The role of invariant natural killer T cells in microbial immunity. J. Infect. Chemother. 19, 560–570 (2013).
van den Elzen, P. et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437, 906–910 (2005).
Allan, L. L. et al. Apolipoprotein-mediated lipid antigen presentation in B cells provides a pathway for innate help by NKT cells. Blood 114, 2411–2416 (2009).
Freigang, S. et al. Scavenger receptors target glycolipids for natural killer T cell activation. J. Clin. Invest. 122, 3943–3954 (2012).
Covarrubias, R., Wilhelm, A. J. & Major, A. S. Specific deletion of LDL receptor-related protein on macrophages has skewed in vivo effects on cytokine production by invariant natural killer T cells. PLoS ONE 9, e102236 (2014).
Van Kaer, L., Parekh, V. V. & Wu, L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 343, 43–55 (2011).
Ait-Oufella, H., Taleb, S., Mallat, Z. & Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 969–979 (2011).
Kleemann, R., Zadelaar, S. & Kooistra, T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc. Res. 79, 360–376 (2008).
Galli, G. et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl Acad. Sci. USA 104, 3984–3989 (2007).
Tonti, E. et al. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood 113, 370–376 (2009).
Yang, J. Q., Wen, X., Kim, P. J. & Singh, R. R. Invariant NKT cells inhibit autoreactive B cells in a contact- and CD1d-dependent manner. J. Immunol. 186, 1512–1520 (2011).
Curtiss, L. K. & Tobias, P. S. Emerging role of Toll-like receptors in atherosclerosis. J. Lipid Res. 50 (Suppl.), S340–S345 (2009).
Choi, S. H., Sviridov, D. & Miller, Y. I. Oxidized cholesteryl esters and inflammation. Biochim. Biophys. Acta http://dx.doi.org/10.1016/j.bbalip.2016.06.020 (2016).
Rocha, D. M., Caldas, A. P., Oliveira, L. L., Bressan, J. & Hermsdorff, H. H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 244, 211–215 (2016).
Fessler, M. B., Rudel, L. L. & Brown, J. M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 20, 379–385 (2009).
Karlsson, F., Tremaroli, V., Nielsen, J. & Backhed, F. Assessing the human gut microbiota in metabolic diseases. Diabetes 62, 3341–3349 (2013).
Tai, N., Wong, F. S. & Wen, L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev. Endocr. Metab. Disord. 16, 55–65 (2015).
Miele, L. et al. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr. Cardiol. Rep. 17, 120 (2015).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Org, E., Mehrabian, M. & Lusis, A. J. Unraveling the environmental and genetic interactions in atherosclerosis: central role of the gut microbiota. Atherosclerosis 241, 387–399 (2015).
Brown, J. M. & Hazen, S. L. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 66, 343–359 (2015).
Fu, J. et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 117, 817–824 (2015).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Palm, N. W., de Zoete, M. R. & Flavell, R. A. Immune-microbiota interactions in health and disease. Clin. Immunol. 159, 122–127 (2015).
Dowds, C. M., Blumberg, R. S. & Zeissig, S. Control of intestinal homeostasis through crosstalk between natural killer T cells and the intestinal microbiota. Clin. Immunol. 159, 128–133 (2015).
Zhang, H. & Luo, X. M. Control of commensal microbiota by the adaptive immune system. Gut Microbes 6, 156–160 (2015).
Zeissig, S. & Blumberg, R. S. Commensal microbiota and NKT cells in the control of inflammatory diseases at mucosal surfaces. Curr. Opin. Immunol. 25, 690–696 (2013).
Wingender, G. et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 143, 418–428 (2012).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 (2014).
McDonald, B. D., Constantinides, M. G. & Bendelac, A. Polarized effector programs for innate-like thymocytes. Nat. Immunol. 14, 1110–1111 (2013).
Org, E. et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7, 313–322 (2016).
Barral, P., Sanchez-Nino, M. D., van Rooijen, N., Cerundolo, V. & Batista, F. D. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 31, 2378–2390 (2012).
Geissmann, F. et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3, e113 (2005).
Nieuwenhuis, E. E. et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Invest. 119, 1241–1250 (2009).
Erridge, C. Diet, commensals and the intestine as sources of pathogen-associated molecular patterns in atherosclerosis, type 2 diabetes and non-alcoholic fatty liver disease. Atherosclerosis 216, 1–6 (2011).
Getz, G. S., Vanderlaan, P. A. & Reardon, C. A. Natural killer T cells in lipoprotein metabolism and atherosclerosis. Thromb. Haemost. 106, 814–819 (2011).
Lynch, L. et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37, 574–587 (2012).
VanderLaan, P. A. et al. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am. J. Pathol. 170, 1100–1107 (2007).
Martin-Murphy, B. V. et al. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS ONE 9, e80949 (2014).
Subramanian, S. et al. Increased levels of invariant natural killer T lymphocytes worsen metabolic abnormalities and atherosclerosis in obese mice. J. Lipid Res. 54, 2831–2841 (2013).
Nakai, Y. et al. Natural killer T cells accelerate atherogenesis in mice. Blood 104, 2051–2059 (2004).
Tupin, E. et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 199, 417–422 (2004).
Major, A. S. et al. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 24, 2351–2357 (2004).
Braun, N. A. et al. Development of spontaneous anergy in invariant natural killer T cells in a mouse model of dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 30, 1758–1765 (2010).
Rogers, L. et al. Deficiency of invariant V alpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc. Res. 78, 167–174 (2008).
Aslanian, A. M., Chapman, H. A. & Charo, I. F. Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 25, 628–632 (2005).
van Puijvelde, G. H. et al. Effect of natural killer T cell activation on the initiation of atherosclerosis. Thromb. Haemost. 102, 223–230 (2009).
Li, Y. et al. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin- and granzyme B-dependent cytotoxicity. Circ. Res. 116, 245–254 (2015).
Matsuda, J. L. et al. Homeostasis of V alpha 14i NKT cells. Nat. Immunol. 3, 966–974 (2002).
Thorp, E., Subramanian, M. & Tabas, I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur. J. Immunol. 41, 2515–2518 (2011).
Kanellakis, P. et al. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 313–319 (2011).
Pan, Y. et al. The western-type diet induces anti-HMGB1 autoimmunity in Apoe−/− mice. Atherosclerosis 251, 31–38 (2016).
Li, Y. et al. A CD1d-dependent lipid antagonist to NKT cells ameliorates atherosclerosis in ApoE−/− mice by reducing lesion necrosis and inflammation. Cardiovasc. Res. 109, 305–317 (2016).
Wu, L. et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl Acad. Sci. USA 109, E1143–E1152 (2012).
Satoh, M. et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS ONE 7, e30568 (2012).
Strodthoff, D. et al. Lack of invariant natural killer T cells affects lipid metabolism in adipose tissue of diet-induced obese mice. Arterioscler. Thromb. Vasc. Biol. 33, 1189–1196 (2013).
Omar, A., Chatterjee, T. K., Tang, Y., Hui, D. Y. & Weintraub, N. L. Proinflammatory phenotype of perivascular adipocytes. Arterioscler. Thromb. Vasc. Biol. 34, 1631–1636 (2014).
Majesky, M. W. Adventitia and perivascular cells. Arterioscler. Thromb. Vasc. Biol. 35, e31–e35 (2015).
Andoh, Y. et al. Natural killer T cells are required for lipopolysaccharide-mediated enhancement of atherosclerosis in apolipoprotein E-deficient mice. Immunobiology 218, 561–569 (2013).
Siddiqui, S., Visvabharathy, L. & Wang, C. R. Role of group 1 CD1-restricted T cells in infectious disease. Front. Immunol. 6, 337 (2015).
Wun, K. S. et al. Human and mouse type I natural killer T cell antigen receptors exhibit different fine specificities for CD1d-antigen complex. J. Biol. Chem. 287, 39139–39148 (2012).
Cox, D. et al. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE 4, e5325 (2009).
Fox, L. M. et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 7, e1000228 (2009).
Pei, B. et al. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J. Immunol. 186, 1348–1360 (2011).
Maricic, I., Girardi, E., Zajonc, D. M. & Kumar, V. Recognition of lysophosphatidylcholine by type II NKT cells and protection from an inflammatory liver disease. J. Immunol. 193, 4580–4589 (2014).
Bobryshev, Y. V. & Lord, R. S. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J. Histochem. Cytochem. 53, 781–785 (2005).
Chan, W. L. et al. Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ. Res. 96, 675–683 (2005).
Kyriakakis, E. et al. Invariant natural killer T cells: linking inflammation and neovascularization in human atherosclerosis. Eur. J. Immunol. 40, 3268–3279 (2010).
Chatterjee, S. B., Dey, S., Shi, W. Y., Thomas, K. & Hutchins, G. M. Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology 7, 57–65 (1997).
Schulze, H. & Sandhoff, K. Lysosomal lipid storage diseases. Cold Spring Harb. Perspect. Biol. 3, a004804 (2011).
Felley, L. & Gumperz, J. E. Are human iNKT cells keeping tabs on lipidome perturbations triggered by oxidative stress in the blood? Immunogenetics 68, 611–622 (2016).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Christiansen, D. et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 6, e172 (2008).
Weismann, D. & Binder, C. J. The innate immune response to products of phospholipid peroxidation. Biochim. Biophys. Acta 1818, 2465–2475 (2012).
Purcell-Huynh, D. A. et al. Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high-fat diet. J. Clin. Invest. 95, 2246–2257 (1995).
VanderLaan, P. A., Reardon, C. A. & Getz, G. S. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 24, 12–22 (2004).
Acknowledgements
This work is supported by National Institutes of Health grant HL088420.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript, and reviewed/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Getz, G., Reardon, C. Natural killer T cells in atherosclerosis. Nat Rev Cardiol 14, 304–314 (2017). https://doi.org/10.1038/nrcardio.2017.2
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.2
This article is cited by
-
Changes in natural killer and T lymphocyte phenotypes in response to cardiovascular risk management
Scientific Reports (2023)
-
Crosstalk between trace elements and T-cell immunity during early-life health in pigs
Science China Life Sciences (2023)
-
The why and how of adaptive immune responses in ischemic cardiovascular disease
Nature Cardiovascular Research (2022)
-
How the immune system shapes atherosclerosis: roles of innate and adaptive immunity
Nature Reviews Immunology (2022)
-
Immune cell profiling in atherosclerosis: role in research and precision medicine
Nature Reviews Cardiology (2022)