Key Points
-
Clinical trials to test the clinical efficacy of gene therapy in humans are rapidly increasing in number, and confidence about advancing gene therapy to the clinical arena is growing
-
Gene therapy for cardiac diseases is less advanced than in some other clinical fields, and the therapeutic utility of gene therapy in the heart is only now being explored
-
Availability of novel viral vectors, such as the adeno-associated viruses, with an improved safety profile, long-term efficacy, and tissue selectivity has greatly contributed to advancements of cardiac gene therapy
-
Inherited and acquired arrhythmias might be suitable targets for gene therapy because of the lack of highly effective pharmacological treatments and the adverse effects of device therapies
-
The greatest challenge in gene therapy for the treatment of arrhythmias is the possibility that transduction of only a small number of cells might trigger proarrhythmic events
-
In a mouse model of recessive catecholaminergic polymorphic ventricular tachycardia, transduction of 40–50% of cardiomyocytes prevented the development of adrenergically-induced life-threatening arrhythmias
Abstract
Gene therapy to treat electrical dysfunction of the heart is an appealing strategy because of the limited therapeutic options available to manage the most-severe cardiac arrhythmias, such as ventricular tachycardia, ventricular fibrillation, and asystole. However, cardiac genetic manipulation is challenging, given the complex mechanisms underlying arrhythmias. Nevertheless, the growing understanding of the molecular basis of these diseases, and the development of sophisticated vectors and delivery strategies, are providing researchers with adequate means to target specific genes and pathways involved in disorders of heart rhythm. Data from preclinical studies have demonstrated that gene therapy can be successfully used to modify the arrhythmogenic substrate and prevent life-threatening arrhythmias. Therefore, gene therapy might plausibly become a treatment option for patients with difficult-to-manage acquired arrhythmias and for those with inherited arrhythmias. In this Review, we summarize the preclinical studies into gene therapy for acquired and inherited arrhythmias of the atria or ventricles. We also provide an overview of the technical advances in the design of constructs and viral vectors to increase the efficiency and safety of gene therapy and to improve selective delivery to target organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Donahue, J. K. et al. Focal modification of electrical conduction in the heart by viral gene transfer. Nat. Med. 6, 1395–1398 (2000).
Priori, S. G. & Napolitano, C. From catheters to vectors: the dawn of molecular electrophysiology. Nat. Med. 6, 1316–1318 (2000).
Edelberg, J. M., Aird, W. C. & Rosenberg, R. D. Enhancement of murine cardiac chronotropy by the molecular transfer of the human beta2 adrenergic receptor cDNA. J. Clin. Invest. 101, 337–343 (1998).
Meister, G. & Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349 (2004).
Grimm, D. et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441, 537–541 (2006).
McBride, J. L. et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc. Natl Acad. Sci. USA 105, 5868–5873 (2008).
Boudreau, R. L., Martins, I. & Davidson, B. L. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 17, 169–175 (2009).
Hohjoh, H. Disease-causing allele-specific silencing by RNA interference. Pharmaceuticals (Basel) 6, 522–535 (2013).
Mueller, C. et al. Sustained miRNA-mediated knockdown of mutant AAT with simultaneous augmentation of wild-type AAT has minimal effect on global liver miRNA profiles. Mol. Ther. 20, 590–600 (2012).
Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S. & Gregory, P. D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 11, 636–646 (2010).
Joung, J. K. & Sander, J. D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55 (2013).
Lombardo, A. et al. Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat. Methods 8, 861–869 (2011).
Lombardo, A. et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 25, 1298–1306 (2007).
Lovric, J. et al. Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins. Mol. Ther. 20, 2087–2097 (2012).
Chan, F., Hauswirth, W. W., Wensel, T. G. & Wilson, J. H. Efficient mutagenesis of the rhodopsin gene in rod photoreceptor neurons in mice. Nucleic Acids Res. 39, 5955–5966 (2011).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Long, C. et al. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345, 1184–1188 (2014).
Lin, H., Parmacek, M. S., Morle, G., Bolling, S. & Leiden, J. M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation 82, 2217–2221 (1990).
Buttrick, P. M., Kass, A., Kitsis, R. N., Kaplan, M. L. & Leinwand, L. A. Behavior of genes directly injected into the rat heart in vivo. Circ. Res. 70, 193–198 (1992).
Penn, M. S. et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ. Res. 112, 816–825 (2013).
Zangi, L. et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 31, 898–907 (2013).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Zhao, J. et al. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res. Cardiol. 97, 348–358 (2002).
Fleury, S. et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation 107, 2375–2382 (2003).
Niwano, K. et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol. Ther. 16, 1026–1032 (2008).
Di Pasquale, E., Latronico, M. V., Jotti, G. S. & Condorelli, G. Lentiviral vectors and cardiovascular diseases: a genetic tool for manipulating cardiomyocyte differentiation and function. Gene Ther. 19, 642–648 (2012).
Hacein-Bey-Abina, S. et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 348, 255–256 (2003).
Hockemeyer, D. et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 27, 851–857 (2009).
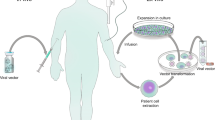
Biffi, A. et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341, 1233158 (2013).
Aiuti, A. et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341, 1233151 (2013).
Palfi, S. et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet 383, 1138–1146 (2014).
Flight, M. H. Trial watch: Clinical trial boost for lentiviral gene therapy. Nat. Rev. Drug Discov. 12, 654 (2013).
Brunner, M. et al. In vivo gene transfer of Kv1.5 normalizes action potential duration and shortens QT interval in mice with long QT phenotype. Am. J. Physiol. Heart Circ. Physiol. 285, H194–H203 (2003).
Amit, G. et al. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation 121, 2263–2270 (2010).
Rosengart, T. K. et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation 100, 468–474 (1999).
French, B. A., Mazur, W., Geske, R. S. & Bolli, R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation 90, 2414–2424 (1994).
Giraud, C., Winocour, E. & Berns, K. I. Site-specific integration by adeno-associated virus is directed by a cellular DNA sequence. Proc. Natl Acad. Sci. USA 91, 10039–10043 (1994).
Flotte, T. R. & Berns, K. I. Adeno-associated virus: a ubiquitous commensal of mammals. Hum. Gene Ther. 16, 401–407 (2005).
Wu, Z., Asokan, A. & Samulski, R. J. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14, 316–327 (2006).
Gao, G. et al. Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum. Gene Ther. 22, 979–984 (2011).
Asokan, A., Schaffer, D. V. & Samulski, R. J. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. 20, 699–708 (2012).
Grieger, J. C. & Samulski, R. J. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J. Virol. 79, 9933–9944 (2005).
England, S. B. et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343, 180–182 (1990).
Chamberlain, J. S. Gene therapy of muscular dystrophy. Hum. Mol. Genet. 11, 2355–2362 (2002).
Liu, M. et al. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol. Ther. 11, 245–256 (2005).
Ghosh, A. & Duan, D. Expanding adeno-associated viral vector capacity: a tale of two vectors. Biotechnol. Genet. Eng. Rev. 24, 165–177 (2007).
Ghosh, A., Yue, Y., Lai, Y. & Duan, D. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol. Ther. 16, 124–130 (2008).
Lai, Y., Yue, Y., Liu, M. & Duan, D. Synthetic intron improves transduction efficiency of trans-splicing adeno-associated viral vectors. Hum. Gene Ther. 17, 1036–1042 (2006).
Koo, T., Popplewell, L., Athanasopoulos, T. & Dickson, G. Triple trans-splicing adeno-associated virus vectors capable of transferring the coding sequence for full-length dystrophin protein into dystrophic mice. Hum. Gene Ther. 25, 98–108 (2014).
Nayak, S. & Herzog, R. W. Progress and prospects: immune responses to viral vectors. Gene Ther. 17, 295–304 (2010).
Mingozzi, F. & High, K. A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122, 23–36 (2013).
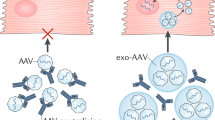
Tan, A., Rajadas, J. & Seifalian, A. M. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv. Drug Deliv. Rev. 65, 357–367 (2013).
Ailawadi, S., Wang, X., Gu, H. & Fan, G. C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim. Biophys. Acta 1852, 1–11 (2015).
Salva, M. Z. et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 15, 320–329 (2007).
Gerolami, R. et al. Gene transfer to hepatocellular carcinoma: transduction efficacy and transgene expression kinetics by using retroviral and lentiviral vectors. Cancer Gene Ther. 7, 1286–1292 (2000).
Denegri, M. et al. Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias. Circ. Res. 110, 663–668 (2012).
Denegri, M. et al. Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age. Circulation 129, 2673–2681 (2014).
Liu, N. et al. Abnormal propagation of calcium waves and ultrastructural remodeling in recessive catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 113, 142–152 (2013).
Geisler, A. et al. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene Ther. 18, 199–209 (2011).
Yang, L. et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc. Natl Acad. Sci. USA 106, 3946–3951 (2009).
Asokan, A. et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 28, 79–82 (2010).
Pulicherla, N. et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 19, 1070–1078 (2011).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Tafuro, S. et al. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovasc. Res. 83, 663–671 (2009).
Jaski, B. E. et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 15, 171–181 (2009).
Jessup, M. et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124, 304–313 (2011).
Katz, M. G., Fargnoli, A. S., Pritchette, L. A. & Bridges, C. R. Gene delivery technologies for cardiac applications. Gene Ther. 19, 659–669 (2012).
Fargnoli, A. S. et al. Cardiac surgical delivery of the sarcoplasmic reticulum calcium ATPase rescues myocytes in ischemic heart failure. Ann. Thorac. Surg. 96, 586–595 (2013).
Igarashi, T. et al. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation 125, 216–225 (2012).
Andrade, J., Khairy, P., Dobrev, D. & Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114, 1453–1468 (2014).
Bauer, A. et al. Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation 110, 3115–3120 (2004).
Lugenbiel, P. et al. Genetic suppression of Gαs protein provides rate control in atrial fibrillation. Basic Res. Cardiol. 107, 265 (2012).
Murata, M., Cingolani, E., McDonald, A. D., Donahue, J. K. & Marbán, E. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ. Res. 95, 398–405 (2004).
Stump, M. R., Gong, Q. & Zhou, Z. Isoform-specific dominant-negative effects associated with hERG1 G628S mutation in long QT syndrome. PLoS ONE 7, e42552 (2012).
Soucek, R. et al. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm 9, 265–272 (2012).
Bikou, O. et al. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc. Res. 92, 218–225 (2011).
Nattel, S., Maguy, A., Le Bouter, S. & Yeh, Y. H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 87, 425–456 (2007).
Firouzi, M. et al. Association of human connexin 40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ. Res. 95, e29–33 (2004).
Thibodeau, I. L. et al. Paradigm of genetic mosaicism and lone atrial fibrillation: physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation 122, 236–244 (2010).
Trappe, K. et al. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre-clinical pilot study. Eur. Heart J. 34, 147–157 (2013).
Lau, D. H. et al. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: an in silico, in vivo, in vitro study. Circulation 119, 19–27 (2009).
Sasano, T., Kelemen, K., Greener, I. D. & Donahue, J. K. Ventricular tachycardia from the healed myocardial infarction scar: validation of an animal model and utility of gene therapy. Heart Rhythm 6 (Suppl.), S91–S97 (2009).
Lyon, A. R. et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ. Arrhythm. Electrophysiol. 4, 362–372 (2011).
Plotnikov, A. N. et al. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation 109, 506–512 (2004).
Bucchi, A. et al. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation 114, 992–999 (2006).
Boink, G. J. et al. HCN2/SkM1 gene transfer into canine left bundle branch induces stable, autonomically responsive biological pacing at physiological heart rates. J. Am. Coll. Cardiol. 61, 1192–1201 (2013).
Miake, J., Marbán, E. & Nuss, H. B. Biological pacemaker created by gene transfer. Nature 419, 132–133 (2002).
Nattel, S. Inward rectifier-funny current balance and spontaneous automaticity: cautionary notes for biologic pacemaker development. Heart Rhythm 5, 1318–1319 (2008).
Valiunas, V. et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J. Physiol. 555, 617–626 (2004).
Cho, H. C., Kashiwakura, Y. & Marbán, E. Creation of a biological pacemaker by cell fusion. Circ. Res. 100, 1112–1115 (2007).
Kapoor, N., Liang, W., Marbán, E. & Cho, H. C. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat. Biotechnol. 31, 54–62 (2013).
Hu, Y. F., Dawkins, J. F., Cho, H. C., Marbán, E. & Cingolani, E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci. Transl. Med. 6, 245ra94 (2014).
International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 431, 931–945 (2004).
Priori, S. G. et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10, 1932–1963 (2013).
London, B. et al. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc. Natl Acad. Sci. USA 95, 2926–2931 (1998).
Kodirov, S. A., Brunner, M., Busconi, L. & Koren, G. Long-term restitution of 4-aminopyridine-sensitive currents in Kv1DN ventricular myocytes using adeno-associated virus-mediated delivery of Kv1.5. FEBS Lett. 550, 74–78 (2003).
Valle, G. et al. Catecholaminergic polymorphic ventricular tachycardia-related mutations R33Q and L167H alter calcium sensitivity of human cardiac calsequestrin. Biochem. J. 413, 291–303 (2008).
Rizzi, N. et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ. Res. 103, 298–306 (2008).
Lompré, A. M. et al. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 121, 822–830 (2010).
Cutler, M. J., Wan, X., Laurita, K. R., Hajjar, R. J. & Rosenbaum, D. S. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ. Arrhythm. Electrophysiol. 2, 686–694 (2009).
Zsebo, K. et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ. Res. 114, 101–108 (2014).
Greenberg, B. et al. Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID 2 trial (Calcium Up-regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease phase 2b). JACC Heart Fail. 2, 84–92 (2014).
Acknowledgements
S.G.P. is also affiliated with the Department of Molecular Medicine, University of Pavia, Pavia, Italy.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript, and reviewed/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
S.G.P. declares that she is a member of the board of directors of Cardiogene Sciences. R.B. declares no competing interests.
Rights and permissions
About this article
Cite this article
Bongianino, R., Priori, S. Gene therapy to treat cardiac arrhythmias. Nat Rev Cardiol 12, 531–546 (2015). https://doi.org/10.1038/nrcardio.2015.61
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2015.61
This article is cited by
-
Biomaterial-induced conversion of quiescent cardiomyocytes into pacemaker cells in rats
Nature Biomedical Engineering (2021)
-
Gene therapy to inhibit CaMKII in CPVT
Nature Reviews Cardiology (2019)
-
Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia
Cell Death & Disease (2016)