Key Points
-
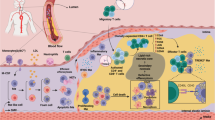
Only M1 proinflammatory and M2 anti-inflammatory macrophages have been described in vitro—however, a wide spectrum of intermediate phenotypes has been identified in in vivo studies
-
Various stimuli (cytokines, lipids and their derivatives, senescent cells, iron) can influence macrophage phenotypes in atherosclerotic lesions
-
Macrophages with different functional phenotypes are likely to perform different roles in the development of atherosclerosis
-
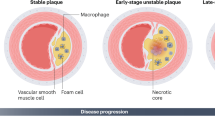
M1 macrophages are associated with symptomatic and unstable plaques, whereas M2 macrophages are particularly abundant in stable zones of the plaque and asymptomatic lesions
-
Modulation of macrophage phenotypes might be a novel strategy for the pharmacological treatment of atherosclerosis
Abstract
Macrophage accumulation within the vascular wall is a hallmark of atherosclerosis. In atherosclerotic lesions, macrophages respond to various environmental stimuli, such as modified lipids, cytokines, and senescent erythrocytes, which can modify their functional phenotypes. The results of studies on human atherosclerotic plaques demonstrate that the relative proportions of macrophage subsets within a plaque might be a better indicator of plaque phenotype and stability than the total number of macrophages. Understanding the function of specific macrophage subsets and their contribution to the composition and growth of atherosclerotic plaques would aid the identification of novel strategies to delay or halt the development of the disease and its associated pathophysiological consequences. However, most studies aimed at characterizing the phenotypes of human macrophages are performed in vitro and, therefore, their functional relevance to human pathology remains uncertain. In this Review, the diverse range of macrophage phenotypes in atherosclerotic lesions and their potential roles in both plaque progression and stability are discussed, with an emphasis on human pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Lusis, A. J. Atherosclerosis. Nature 407, 233–241 (2000).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Libby, P., Aikawa, M. & Schonbeck, U. Cholesterol and atherosclerosis. Biochim. Biophys. Acta 1529, 299–309 (2000).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 (2010).
Waldo, S. W. et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 172, 1112–1126 (2008).
Bouhlel, M. A. et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 (2007).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Gordon, S. & Taylor, P. R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Jenkins, S. J. et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Jenkins, S. J. et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J. Exp. Med. 210, 2477–2491 (2013).
Verreck, F. A. et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco) acteria. Proc. Natl Acad. Sci. USA 101, 4560–4565 (2004).
Mosser, D. M. The many faces of macrophage activation. J. Leukoc. Biol. 73, 209–212 (2003).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Anderson, C. F., Gerber, J. S. & Mosser, D. M. Modulating macrophage function with IgG immune complexes. J. Endotoxin. Res. 8, 477–481 (2002).
Zizzo, G., Hilliard, B. A., Monestier, M. & Cohen, P. L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J. Immunol. 189, 3508–3520 (2012).
Jetten, N. et al. Anti-inflammatory M2, but not proinflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17, 109–118 (2014).
Lee, C. G. et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1 . J. Exp. Med. 194, 809–821 (2001).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Martinez, F. O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 (2006).
Porcheray, F. et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489 (2005).
Lee, S. et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326 (2011).
Feig, J. E. et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl Acad. Sci. USA 108, 7166–7171 (2011).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
van Tits, L. J. et al. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis 214, 345–349 (2011).
Hirose, K. et al. Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids Health Dis. 10, 1 (2011).
Bae, Y. S. et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 104, 210–218 (2009).
Fang, L. et al. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: macrophage binding and activation. J. Biol. Chem. 285, 32343–32351 (2010).
Sottero, B. et al. Expression and synthesis of TGFβ1 is induced in macrophages by 9-oxononanoyl cholesterol, a major cholesteryl ester oxidation product. Biofactors 24, 209–216 (2005).
Hughes, J. E. et al. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 102, 950–958 (2008).
Serhan, C. N. et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 206, 15–23 (2009).
Titos, E. et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 187, 5408–5418 (2011).
Mitchell, P. L. & McLeod, R. S. Conjugated linoleic acid and atherosclerosis: studies in animal models. Biochem. Cell Biol. 86, 293–301 (2008).
McCarthy, C. et al. IL-10 mediates the immunoregulatory response in conjugated linoleic acid-induced regression of atherosclerosis. FASEB J. 27, 499–510 (2013).
Kadl, A. et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 107, 737–746 (2010).
Kadl, A. et al. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic. Biol. Med. 51, 1903–1909 (2011).
Kolodgie, F. D. et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 349, 2316–2325 (2003).
Kockx, M. M. et al. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 440–446 (2003).
Ganz, T. Macrophages and systemic iron homeostasis. J. Innate. Immun. 4, 446–453 (2012).
Finn, A. V. et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J. Am. Coll. Cardiol. 59, 166–177 (2012).
Nielsen, M. J., Moller, H. J. & Moestrup, S. K. Hemoglobin and heme scavenger receptors. Antioxid. Redox Signal. 12, 261–273 (2010).
Philippidis, P. et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 94, 119–126 (2004).
Landis, R. C., Philippidis, P., Domin, J., Boyle, J. J. & Haskard, D. O. Haptoglobin genotype-dependent anti-inflammatory signaling in CD163+ Macrophages. Int. J. Inflam. 2013, 980327 (2013).
Bories, G. et al. Liver X receptor (LXR) activation stimulates iron export in human alternative macrophages. Circ. Res. 113, 1196–1205 (2013).
Recalcati, S. et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 40, 824–835 (2010).
Corna, G. et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 95, 1814–1822 (2010).
Chinetti-Gbaguidi, G. et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 108, 985–995 (2011).
Boyle, J. J. et al. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ. Res. 110, 20–33 (2012).
Wan, X. et al. 5′-AMP-activated protein kinase-activating transcription factor 1 cascade modulates human monocyte-derived macrophages to atheroprotective functions in response to heme or metformin. Arterioscler. Thromb. Vasc. Biol. 33, 2470–2480 (2013).
Boyle, J. J. et al. Heme induces heme oxygenase 1 via Nrf2: role in the homeostatic macrophage response to intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 31, 2685–2691 (2011).
Spann, N. J. et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012).
Sindrilaru, A. et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 121, 985–997 (2012).
Wolfs, I. M., Donners, M. M. & de Winther, M. P. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb. Haemost. 106, 763–771 (2011).
Brocheriou, I. et al. Antagonistic regulation of macrophage phenotype by M-CSF and GM-CSF: implication in atherosclerosis. Atherosclerosis 214, 316–324 (2011).
Plenz, G., Koenig, C., Severs, N. J. & Robenek, H. Smooth muscle cells express granulocyte-macrophage colony-stimulating factor in the undiseased and atherosclerotic human coronary artery. Arterioscler. Thromb. Vasc. Biol. 17, 2489–2499 (1997).
Pitsilos, S. et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb. Haemost. 90, 1112–1120 (2003).
Gleissner, C. A. & Ley, K. CXCL4 in atherosclerosis: possible roles in monocyte arrest and macrophage foam cell formation. Thromb. Haemost. 98, 917–918 (2007).
Gleissner, C. A., Shaked, I., Little, K. M. & Ley, K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J. Immunol. 184, 4810–4818 (2010).
Gleissner, C. A. et al. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ. Res. 106, 203–211 (2010).
Erbel, C. et al. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+in vitro and in vivo. Innate Immun. (in press).
Stoger, J. L. et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 225, 461–468 (2012).
Cho, K. Y. et al. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J. Stroke Cerebrovasc. Dis. 22, 910–918 (2013).
Shaikh, S. et al. Macrophage subtypes in symptomatic carotid artery and femoral artery plaques. Eur. J. Vasc. Endovasc. Surg. 44, 491–497 (2012).
Barlis, P., Serruys, P. W., Devries, A. & Regar, E. Optical coherence tomography assessment of vulnerable plaque rupture: predilection for the plaque 'shoulder'. Eur. Heart J. 29, 2023 (2008).
Khallou-Laschet, J. et al. Macrophage plasticity in experimental atherosclerosis. PLoS ONE 5, e8852 (2010).
Hirata, Y. et al. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int. Heart J. 52, 139–142 (2011).
Hirata, Y. et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 58, 248–255 (2011).
Cougoule, C. et al. Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur. J. Cell Biol. 91, 938–949 (2012).
Lee, C. W. et al. Macrophage heterogeneity of culprit coronary plaques in patients with acute myocardial infarction or stable angina. Am. J. Clin. Pathol. 139, 317–322 (2013).
Huang, W. C., Sala-Newby, G. B., Susana, A., Johnson, J. L. & Newby, A. C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS ONE 7, e42507 (2012).
Feig, J. E. et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS ONE 7, e39790 (2012).
Feig, J. E. et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123, 989–998 (2011).
Gui, T., Shimokado, A., Sun, Y., Akasaka, T. & Muragaki, Y. Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery. Mediators Inflamm. 2012, 693083 (2012).
Acknowledgements
The authors acknowledge grants from the Fondation de France (G.C.-G.), the Fondation pour la Recherche Médicale (G.C.-G.), and the Transatlantic Leducq HDL Network (B.S.). B.S. is a member of the Institut Universitaire de France.
Author information
Authors and Affiliations
Contributions
G.C.-G. and S.C. researched data for the article. S.C. and B.S. substantially contributed to the discussion of content. G.C.-G. wrote the manuscript. B.S. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chinetti-Gbaguidi, G., Colin, S. & Staels, B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol 12, 10–17 (2015). https://doi.org/10.1038/nrcardio.2014.173
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2014.173
This article is cited by
-
GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis
Nature Communications (2023)
-
Sitosterol-rich Digera muricata against 7-ketocholesterol and lipopolysaccharide-mediated atherogenic responses by modulating NF-ΚB/iNOS signalling pathway in macrophages
3 Biotech (2023)
-
New insights into macrophage subsets in atherosclerosis
Journal of Molecular Medicine (2022)
-
Atherosclerosis: nexus of vascular dynamics and cellular cross talks
Molecular and Cellular Biochemistry (2022)
-
Mettl14 mediates the inflammatory response of macrophages in atherosclerosis through the NF-κB/IL-6 signaling pathway
Cellular and Molecular Life Sciences (2022)