Key Points
-
The lymphatic vasculature is essential for immune function, tissue fluid homeostasis and the absorption of dietary fat.
-
The process of lymphangiogenesis involves the formation of new lymphatic vessels from pre-existing lymphatics; this occurs during embryonic development, wound healing and in various pathological contexts, including cancer.
-
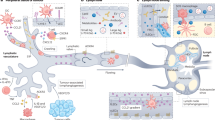
Tumour cells and cells of the tumour microenvironment produce growth factors that promote lymphangiogenesis from initial lymphatics, as well as the enlargement of initial and collecting lymphatic vessels in and around solid tumours. The enlargement of collecting lymphatics can involve remodelling of these vessels by smooth muscle cells.
-
Lymphangiogenic factors (such as vascular endothelial growth factor C (VEGFC) and VEGFD) can induce the metastatic spread of tumours in mouse models of cancer.
-
Clinicopathological studies have shown that the production of lymphangiogenic factors, lymphangiogenesis and lymphatic remodelling can correlate with cancer progression.
-
Lymphatic vessels provide a therapeutic target for modulating the immune response to cancer and restricting metastasis; clinical trials of agents that target lymphangiogenic signalling pathways are underway.
-
Mouse models and genome-wide functional screening approaches might identify further important signalling pathways in tumour lymphangiogenesis that could be potential diagnostic and therapeutic targets.
Abstract
The generation of new lymphatic vessels through lymphangiogenesis and the remodelling of existing lymphatics are thought to be important steps in cancer metastasis. The past decade has been exciting in terms of research into the molecular and cellular biology of lymphatic vessels in cancer, and it has been shown that the molecular control of tumour lymphangiogenesis has similarities to that of tumour angiogenesis. Nevertheless, there are significant mechanistic differences between these biological processes. We are now developing a greater understanding of the specific roles of distinct lymphatic vessel subtypes in cancer, and this provides opportunities to improve diagnostic and therapeutic approaches that aim to restrict the progression of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tuttle, T. M. Technical Advances In Sentinel Lymph Node Biopsy For Breast Cancer. Am. Surg. 70, 407–413 (2004).
Stacker, S. A. et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature Med. 7, 186–191 (2001).
Mandriota, S. J. et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20, 672–682 (2001).
Skobe, M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Med. 7, 192–198 (2001).
Karpanen, T. et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61, 1786–1790 (2001).
Karnezis, T. et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 21, 181–195 (2012). References 2–6 show the ability of VEGFC and VEGFD to promote tumour angiogenesis, lymphangiogenesis and lymphatic metastasis. They also show the potential of therapeutic approaches that target these growth factors and associated processes.
Gogineni, A. et al. Inhibition of VEGF-C modulates distal lymphatic remodeling and secondary metastasis. PLoS ONE 8, e68755 (2013). References 6 and 7 examine the molecular and cellular mechanisms whereby collecting lymphatics can control the rate of tumour metastasis, both proximal and distal to the sentinel lymph node.
Hirakawa, S. et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109, 1010–1017 (2007).
Achen, M. G., Mann, G. B. & Stacker, S. A. Targeting lymphangiogenesis to prevent tumour metastasis. Br. J. Cancer 94, 1355–1360 (2006).
Mumprecht, V. et al. In vivo imaging of inflammation- and tumor-induced lymph node lymphangiogenesis by immuno-positron emission tomography. Cancer Res. 70, 8842–8851 (2010).
Asellius, G. De Lacteibus sive lacteis venis Quarto Vasorum Mesaroicum genere novo invente Gasp. Asellii Cremonensis Antomici Ticiensis Qua Sententiae Anatomicae multae, nel perperam receptae illustrantur. (Mediolani, 1627).
Gerli, R., Solito, R., Weber, E. & Agliano, M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology 33, 148–157 (2000).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007). This manuscript reports on the specialized junctions used by LECs to allow them to participate in fluid transport while maintaining their integrity.
Miteva, D. O. et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 106, 920–931 (2010).
Alitalo, K. The lymphatic vasculature in disease. Nature Med. 17, 1371–1380 (2011). This is a comprehensive review of the role of lymphatics and the lymphatic endothelium in human disease.
Tammela, T. & Alitalo, K. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140, 460–476 (2010).
Schulte-Merker, S., Sabine, A. & Petrova, T. V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–618 (2011).
Makinen, T. et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 20, 4762–4773 (2001). This study reports the isolation and biological characterization of LECs, including signalling pathways for growth, survival and migration.
Shibuya, M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 153, 13–19 (2013).
Karpanen, T. et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 20, 1462–1472 (2006).
Harris, N. C. et al. Proteolytic processing of vascular endothelial growth factor-D is essential for its capacity to promote the growth and spread of cancer. FASEB J. 25, 2615–2625 (2011).
Chung, A. S. & Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 27, 563–584 (2011).
Tammela, T. et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nature Cell Biol. 13, 1202–1213 (2011).
Zheng, W. et al. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 118, 1154–1162 (2011).
Xu, Y. et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 188, 115–130 (2010).
Kriehuber, E. et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 194, 797–808 (2001).
Podgrabinska, S. et al. Molecular characterization of lymphatic endothelial cells. Proc. Natl Acad. Sci. USA 99, 16069–16074 (2002).
Hirakawa, S. et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 162, 575–586 (2003).
Wick, N. et al. Transcriptomal comparison of human dermal lymphatic endothelial cells ex vivo and in vitro. Physiol. Genomics 28, 179–192 (2007).
Kawai, Y. et al. Heterogeneity in immunohistochemical, genomic, and biological properties of human lymphatic endothelial cells between initial and collecting lymph vessels. Lymphat. Res. Biol. 6, 15–27 (2008).
Herbert, S. P. & Stainier, D. Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nature Rev. Mol. Cell. Biol. 12, 551–564 (2011).
Koltowska, K., Betterman, K. L., Harvey, N. L. & Hogan, B. M. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development 140, 1857–1870 (2013).
Hogan, B. M. et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nature Genet. 41, 396–398 (2009).
Farnsworth, R. H., Achen, M. G. & Stacker, S. A. Lymphatic endothelium: an important interactive surface for malignant cells. Pulm. Pharmacol. Ther. 19, 51–60 (2006).
Dadras, S. S. et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am. J. Pathol. 162, 1951–1960 (2003). This paper helps to establish that tumour lymphangiogenesis has prognostic importance in human cancers: in this case, cutaneous melanoma.
Leu, A. J., Berk, D. A., Lymboussaki, A., Alitalo, K. & Jain, R. K. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 60, 4324–4327 (2000).
Padera, T. P. et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296, 1883–1886 (2002).
Shayan, R. et al. Tumor location and nature of lymphatic vessels are key determinants of cancer metastasis. Clin. Exp. Metastasis 30, 345–356 (2013).
Wong, S. Y. et al. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 65, 9789–9798 (2005).
Karpanen, T. & Alitalo, K. Lymphatic vessels as targets of tumor therapy. J. Exp. Med. 194, F37–F42 (2001).
Krishnan, J. et al. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 63, 713–722 (2003).
Ji, R. C. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: new insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 25, 677–694 (2006).
Hoshida, T. et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 66, 8065–8075 (2006). This study uses mouse models and intravital microscopy to show that factors such as VEGFC can increase the delivery of cancer cells to lymph nodes, through mechanisms such as hyperplasia of peritumoural lymphatic vessels.
He, Y. et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 65, 4739–4746 (2005).
Joukov, V. et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898–3911 (1997).
Siegfried, G. et al. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J. Clin. Invest. 111, 1723–1732 (2003).
Stacker, S. A. et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 274, 32127–32136 (1999).
Baldwin, M. E. et al. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J. Biol. Chem. 276, 44307–44314 (2001).
McColl, B. K. et al. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J. Exp. Med. 198, 863–868 (2003).
McColl, B. K. et al. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 21, 1088–1098 (2007). References 45–50 describe the important role that proteolysis has in the activation of key lymphangiogenic growth factors.
Schoppmann, S. F. et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 161, 947–956 (2002).
Kerjaschki, D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Invest. 115, 2316–2319 (2005).
Debinski, W. et al. VEGF-D is an X-linked/AP-1 regulated putative onco-angiogen in human glioblastoma multiforme. Mol. Med. 7, 598–608 (2001).
Tvorogov, D. et al. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell 18, 630–640 (2010).
Pytowski, B. et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J. Natl Cancer Inst. 97, 14–21 (2005).
Roberts, N. et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 66, 2650–2657 (2006).
He, Y. et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl Cancer Inst. 94, 819–825 (2002).
Lin, J. et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 65, 6901–6909 (2005).
Laakkonen, P. et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 67, 593–599 (2007).
Caunt, M. et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 13, 331–342 (2008).
Niederleithner, H. et al. Wnt1 is anti-lymphangiogenic in a melanoma mouse model. J. Invest. Dermatol. 132, 2235–2244 (2012).
Su, J. L. et al. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 64, 554–564 (2004).
Timoshenko, A. V., Chakraborty, C., Wagner, G. F. & Lala, P. K. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br. J. Cancer 94, 1154–1163 (2006).
Soumaoro, L. T. et al. Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its association with lymph node metastasis. Dis. Colon Rectum 49, 392–398 (2006).
Kubo, H. et al. Host prostaglandin EP3 receptor signaling relevant to tumor-associated lymphangiogenesis. Biomed. Pharmacother. 64, 101–106 (2010).
Hosono, K. et al. Roles of prostaglandin E2-EP3/EP4 receptor signaling in the enhancement of lymphangiogenesis during fibroblast growth factor-2-induced granulation formation. Arterioscler. Thromb. Vasc. Biol. 31, 1049–1058 (2011).
Zhang, X. H. et al. Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer 8, 4 (2008).
Bjorndahl, M. A. et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 65, 9261–9268 (2005).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Fagiani, E., Lorentz, P., Kopfstein, L. & Christofori, G. Angiopoietin-1 and -2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 71, 5717–5727 (2011).
Holopainen, T. et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J. Natl Cancer Inst. 104, 461–475 (2012).
Cao, R. et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc. Natl Acad. Sci. USA 109, 15894–15899 (2012).
Cao, R. et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6, 333–345 (2004).
Schito, L. et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc. Natl Acad. Sci. USA 109, E2707–E2716 (2012).
Yoo, Y. A. et al. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 71, 7061–7070 (2011).
Lee, A. S. et al. Erythropoietin induces lymph node lymphangiogenesis and lymph node tumor metastasis. Cancer Res. 71, 4506–4517 (2011).
Karpinich, N. O. et al. Adrenomedullin gene dosage correlates with tumor and lymph node lymphangiogenesis. FASEB J. 27, 590–600 (2013).
Nagahashi, M. et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 72, 726–735 (2012).
Bracher, A. et al. Epidermal growth factor facilitates melanoma lymph node metastasis by influencing tumor lymphangiogenesis. J. Invest. Dermatol. 133, 230–238 (2013).
Patel, V. et al. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 71, 7103–7112 (2011).
Oka, M. et al. Inhibition of endogenous TGF-β signaling enhances lymphangiogenesis. Blood 111, 4571–4579 (2008).
Garmy-Susini, B. et al. Integrin α4β1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 70, 3042–3051 (2010).
Iwasaki, T. et al. Deletion of tetraspanin CD9 diminishes lymphangiogenesis in vivo and in vitro. J. Biol. Chem. 288, 2118–2131 (2013).
Clasper, S. et al. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 68, 7293–7303 (2008).
Forster, R., Davalos-Misslitz, A. C. & Rot, A. CCR7 and its ligands: balancing immunity and tolerance. Nature Rev. Immunol. 8, 362–371 (2008).
Shields, J. D. et al. Chemokine-mediated migration of melanoma cells towards lymphatics — a mechanism contributing to metastasis. Oncogene 26, 2997–3005 (2007).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Kawada, K. et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 64, 4010–4017 (2004).
Uchida, D. et al. Acquisition of lymph node, but not distant metastatic potentials, by the overexpression of CXCR4 in human oral squamous cell carcinoma. Lab. Invest. 84, 1538–1546 (2004).
Kawada, K. et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 26, 4679–4688 (2007).
Gunther, K. et al. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int. J. Cancer 116, 726–733 (2005).
Cabioglu, N. et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin. Cancer Res. 11, 5686–5693 (2005).
Mashino, K. et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 62, 2937–2941 (2002).
Schimanski, C. C. et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br. J. Cancer 95, 210–217 (2006).
Ishikawa, T. et al. CXCR4 expression is associated with lymph-node metastasis of oral squamous cell carcinoma. Int. J. Oncol. 28, 61–66 (2006).
Salvucci, O. et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res. Treat. 97, 275–283 (2006).
Hirakawa, S. et al. Nodal lymphangiogenesis and metastasis: role of tumor-induced lymphatic vessel activation in extramammary Paget's disease. Am. J. Pathol. 175, 2235–2248 (2009).
Shields, J. D. et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538 (2007). This study shows how lymphatic drainage influences interstitial fluid flow and this can direct tumour cell migration through an autocrine CCR7 signalling pathway.
Munson, J. M., Bellamkonda, R. V. & Swartz, M. A. Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res. 73, 1536–1546 (2013).
Issa, A., Le, T. X., Shoushtari, A. N., Shields, J. D. & Swartz, M. A. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 69, 349–357 (2009).
Das, S. et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J. Exp. Med. 210, 1509–1528 (2013).
Wirzenius, M. et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J. Exp. Med. 204, 1431–1440 (2007).
Hirakawa, S. From tumor lymphangiogenesis to lymphvascular niche. Cancer Sci. 100, 983–989 (2009).
Coultas, L., Chawengsaksophak, K. & Rossant, J. Endothelial cells and VEGF in vascular development. Nature 438, 937–945 (2005).
Farnsworth, R. H. et al. A role for bone morphogenic protein-4 in vascular endothelial growth factor-D mediated tumor growth, metastasis and vessel remodelling. Cancer Res. 71, 6547–6557 (2011).
Kim, M. et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 70, 10411–10421 (2010).
Lyden, D. et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 7, 1194–1201 (2001).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Tewalt, E. F., Cohen, J. N., Rouhani, S. J. & Engelhard, V. H. Lymphatic endothelial cells — key players in regulation of tolerance and immunity. Front. Immunol. 3, 305 (2012).
Tewalt, E. F. et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120, 4772–4782 (2012).
Lund, A. W. et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 1, 191–199 (2012). This paper describes how VEGFC is able to promote immune tolerance in a melanoma model and that the lymphatic endothelium might be a target for immunomodulation in the local microenvironment.
Swartz, M. A. & Lund, A. W. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nature Rev. Cancer 12, 210–219 (2012). This review brings together several disparate areas — mechanobiology, fluid dynamics, cell biology and the immune system — to try and understand the interplay between these factors in the role of the lymphatics in cancer progression.
Wang, J. et al. Lymphatic microvessel density and vascular endothelial growth factor-C and -D as prognostic factors in breast cancer: a systematic review and meta-analysis of the literature. Mol. Biol. Rep. 39, 11153–11165 (2012).
Liersch, R., Hirakawa, S., Berdel, W. E., Mesters, R. M. & Detmar, M. Induced lymphatic sinus hyperplasia in sentinel lymph nodes by VEGF-C as the earliest premetastatic indicator. Int. J. Oncol. 41, 2073–2078 (2012).
Fisher, B. et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 52, 1551–1557 (1983).
Foster, R. S. Jr. The biologic and clinical significance of lymphatic metastases in breast cancer. Surg. Oncol. Clin. N. Am. 5, 79–104 (1996).
Wong, S. L. et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J. Clin. Oncol. 30, 2912–2918 (2012).
D'Angelo-Donovan, D. D., Dickson-Witmer, D. & Petrelli, N. J. Sentinel lymph node biopsy in breast cancer: a history and current clinical recommendations. Surg. Oncol. 21, 196–200 (2012).
Harouaka, R. A., Nisic, M. & Zheng, S. Y. Circulating tumor cell enrichment based on physical properties. J. Lab. Autom. http://dx.doi.org/10.1177/2211068213494391 (2013).
Gonzalez-Masia, J. A., Garcia-Olmo, D. & Garcia-Olmo, D. C. Circulating nucleic acids in plasma and serum (CNAPS): applications in oncology. Onco. Targets Ther. 6, 819–832 (2013).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Med. 18, 883–891 (2012).
Ran, S., Volk, L., Hall, K. & Flister, M. J. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 17, 229–251 (2010). This is a comprehensive review of lymphangiogenesis and lymphatic metastasis in relation to breast cancer.
Achen, M. G., McColl, B. K. & Stacker, S. A. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell 7, 121–127 (2005).
Alitalo, A. & Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 31, 4499–4508 (2012).
Doeden, K. et al. Lymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasis. J. Cutan. Pathol. 36, 772–780 (2009).
Saad, R. S. et al. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod. Pathol. 19, 1317–1323 (2006).
Van der Auwera, I. et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin. Cancer Res. 10, 7965–7971 (2004).
Williams, C. S. et al. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J. Pathol. 200, 195–206 (2003).
Sipos, B. et al. Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis. J. Pathol. 207, 301–312 (2005).
Stacker, S. A., Williams, R. A. & Achen, M. G. Lymphangiogenic growth factors as markers of tumor metastasis. APMIS 112, 539–549 (2004).
Yonemura, Y. et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin. Cancer Res. 5, 1823–1829 (1999).
Yokoyama, Y. et al. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin. Cancer Res. 9, 1361–1369 (2003).
White, J. D. et al. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 62, 1669–1675 (2002).
Proulx, S. T. et al. Expansion of the lymphatic vasculature in cancer and inflammation: New opportunities for in vivo imaging and drug delivery. J. Control Release 172, 550–557 (2013).
Mumprecht, V. & Detmar, M. In vivo imaging of lymph node lymphangiogenesis by immuno-positron emission tomography. Methods Mol. Biol. 961, 129–140 (2013).
Mumprecht, V., Roudnicky, F. & Detmar, M. Inflammation-induced lymph node lymphangiogenesis is reversible. Am. J. Pathol. 180, 874–879 (2012).
Veikkola, T. et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 20, 1223–1231 (2001).
Dadras, S. S. et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod. Pathol. 18, 1232–1242 (2005).
Tammela, T. et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454, 656–660 (2008).
Siekmann, A. F. & Lawson, N. D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781–784 (2007).
Madsen, C. D. & Sahai, E. Cancer dissemination-lessons from leukocytes. Dev. Cell 19, 13–26 (2010).
Neal, J. & Wakelee, H. AMG-386, a selective angiopoietin-1/-2-neutralizing peptibody for the potential treatment of cancer. Curr. Opin. Mol. Ther. 12, 487–495 (2010).
Sun, W. & Haller, D. G. Adjuvant therapy of colon cancer. Semin. Oncol. 32, 95–102 (2005).
Matsumoto, M. et al. Signaling for lymphangiogenesis via VEGFR-3 is required for the early events of metastasis. Clin. Exp. Metastasis 30, 819–832 (2013).
Banerji, S. et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801 (1999).
Breiteneder-Geleff, S. et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 151, 1141–1152 (1997).
Makinen, T. et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 19, 397–410 (2005).
Schacht, V. et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am. J. Pathol. 166, 913–921 (2005).
Boone, B. et al. The role of VEGF-C staining in predicting regional metastasis in melanoma. Virchows Arch. 453, 257–265 (2008).
Schietroma, C. et al. Vascular endothelial growth factor-C expression correlates with lymph node localization of human melanoma metastases. Cancer 98, 789–797 (2003).
Tobler, N. E. & Detmar, M. Tumor and lymph node lymphangiogenesis — impact on cancer metastasis. J. Leukoc. Biol. 80, 691–696 (2006).
Xu, X. et al. Lymphatic invasion is independently prognostic of metastasis in primary cutaneous melanoma. Clin. Cancer Res. 18, 229–237 (2012).
Raica, M., Cimpean, A. M., Ceausu, R. & Ribatti, D. Lymphatic microvessel density, VEGF-C, and VEGFR-3 expression in different molecular types of breast cancer. Anticancer Res. 31, 1757–1764 (2011).
Mohammed, R. A. et al. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br. J. Cancer 96, 1092–1100 (2007).
Gu, Y., Qi, X. & Guo, S. Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: a retrospective study of 61 cases. Clin. Exp. Metastasis 25, 717–725 (2008).
Koyama, Y. et al. Vascular endothelial growth factor-C and vascular endothelial growth factor-d messenger RNA expression in breast cancer: association with lymph node metastasis. Clin. Breast Cancer 4, 354–360 (2003).
Gisterek, I. et al. Evaluation of prognostic value of VEGF-C and VEGF-D in breast cancer — 10 years follow-up analysis. Anticancer Res. 27, 2797–2802 (2007).
Nakamura, Y. et al. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res. Treat. 91, 125–132 (2005).
Agarwal, B., Saxena, R., Morimiya, A., Mehrotra, S. & Badve, S. Lymphangiogenesis does not occur in breast cancer. Am. J. Surg. Pathol. 29, 1449–1455 (2005).
Bono, P. et al. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin. Cancer Res. 10, 7144–7149 (2004).
Mohammed, R. A., Ellis, I. O., Elsheikh, S., Paish, E. C. & Martin, S. G. Lymphatic and angiogenic characteristics in breast cancer: morphometric analysis and prognostic implications. Breast Cancer Res. Treat. 113, 261–273 (2009).
Marinho, V. F., Metze, K., Sanches, F. S., Rocha, G. F. & Gobbi, H. Lymph vascular invasion in invasive mammary carcinomas identified by the endothelial lymphatic marker D2-40 is associated with other indicators of poor prognosis. BMC Cancer 8, 64 (2008).
Travagli, J. P. et al. Sentinel lymphadenectomy without systematic axillary dissection in breast cancer patients: predictors of non-sentinel lymph node metastasis. Eur. J. Surg. Oncol. 29, 403–406 (2003).
Arnaout-Alkarain, A., Kahn, H. J., Narod, S. A., Sun, P. A. & Marks, A. N. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod. Pathol. 20, 183–191 (2007).
Moehler, M. et al. VEGF-D expression correlates with colorectal cancer aggressiveness and is downregulated by cetuximab. World J. Gastroenterol. 14, 4156–4167 (2008).
Fukunaga, S. et al. Association between expression of vascular endothelial growth factor C, chemokine receptor CXCR4 and lymph node metastasis in colorectal cancer. Oncology 71, 204–211 (2006).
Doekhie, F. S. et al. Sialyl Lewis X expression and lymphatic microvessel density in primary tumors of node-negative colorectal cancer patients predict disease recurrence. Cancer Microenviron. 1, 141–151 (2008).
Matsumoto, K. et al. Lymphatic microvessel density is an independent prognostic factor in colorectal cancer. Dis. Colon Rectum 50, 308–314 (2007).
Kaneko, I. et al. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis. Colon Rectum 50, 13–21 (2007).
Barresi, V., Reggiani-Bonetti, L., Di Gregorio, C., De Leon, M. P. & Barresi, G. Lymphatic vessel density and its prognostic value in stage I colorectal carcinoma. J. Clin. Pathol. 64, 6–12 (2011).
Schoppmann, A. et al. Comparison of lymphangiogenesis between primary colorectal cancer and corresponding liver metastases. Anticancer Res. 31, 4605–4611 (2011).
Akagi, Y., Adachi, Y., Ohchi, T., Kinugasa, T. & Shirouzu, K. Prognostic impact of lymphatic invasion of colorectal cancer: a single-center analysis of 1,616 patients over 24 years. Anticancer Res. 33, 2965–2970 (2013).
Barresi, V. et al. Immunohistochemical assessment of lymphovascular invasion in stage I colorectal carcinoma: prognostic relevance and correlation with nodal micrometastases. Am. J. Surg. Pathol. 36, 66–72 (2012).
Lim, S. B. et al. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis. Colon Rectum 53, 377–384 (2010).
Kojima, H. et al. Clinical significance of vascular endothelial growth factor-C and vascular endothelial growth factor receptor 3 in patients with T1 lung adenocarcinoma. Cancer 104, 1668–1677 (2005).
Ogawa, E. et al. Clinical significance of VEGF-C status in tumour cells and stromal macrophages in non-small cell lung cancer patients. Br. J. Cancer 91, 498–503 (2004).
Takanami, I. Lymphatic microvessel density using D2-40 is associated with nodal metastasis in non-small cell lung cancer. Oncol. Rep. 15, 437–442 (2006).
Adachi, Y. et al. Lymphatic vessel density in pulmonary adenocarcinoma immunohistochemically evaluated with anti-podoplanin or anti-D2-40 antibody is correlated with lymphatic invasion or lymph node metastases. Pathol. Int. 57, 171–177 (2007).
Renyi-Vamos, F. et al. Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non-small cell lung cancer. Clin. Cancer Res. 11, 7344–7353 (2005).
Higgins, K. A. et al. Lymphovascular invasion in non-small-cell lung cancer: implications for staging and adjuvant therapy. J. Thorac. Oncol. 7, 1141–1147 (2012).
Schmid, K., Birner, P., Gravenhorst, V., End, A. & Geleff, S. Prognostic value of lymphatic and blood vessel invasion in neuroendocrine tumors of the lung. Am. J. Surg. Pathol. 29, 324–328 (2005).
Hanagiri, T. et al. Prognostic significance of lymphovascular invasion for patients with stage I non-small cell lung cancer. Eur. Surg. Res. 47, 211–217 (2011).
Hajrasouliha, A. R. et al. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 53, 1244–1250 (2012).
Achen, M. G. et al. Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem. 267, 2505–2515 (2000).
Davydova, N., Roufail, S., Streltsov, V. A., Stacker, S. A. & Achen, M. G. The VD1 neutralizing antibody to vascular endothelial growth factor-D: binding epitope and relationship to receptor binding. J. Mol. Biol. 407, 581–593 (2011).
Kashima, K. et al. Inhibition of lymphatic metastasis in neuroblastoma by a novel neutralizing antibody to vascular endothelial growth factor-D. Cancer Sci. 103, 2144–2152 (2012).
Makinen, T. et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nature Med. 7, 199–205 (2001).
Albuquerque, R. J. et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nature Med. 15, 1023–1030 (2009).
Persaud, K. et al. Involvement of the VEGF receptor 3 in tubular morphogenesis demonstrated with a human anti-human VEGFR-3 monoclonal antibody that antagonizes receptor activation by VEGF-C. J. Cell Sci. 117, 2745–2756 (2004).
Procopio, G. et al. Experience with sorafenib in the treatment of advanced renal cell carcinoma. Ther. Adv. Urol. 4, 303–313 (2012).
Verweij, J. & Sleijfer, S. Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert. Opin. Pharmacother. 14, 929–935 (2013).
Mankal, P. & O'Reilly, E. Sunitinib malate for the treatment of pancreas malignancies — where does it fit? Expert. Opin. Pharmacother. 14, 783–792 (2013).
Kodera, Y. et al. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res. 13, R66 (2011).
Grunwald, V. & Merseburger, A. S. Axitinib for the treatment of patients with advanced metastatic renal cell carcinoma (mRCC) after failure of prior systemic treatment. Onco. Targets Ther. 5, 111–117 (2012).
Davis, S. L., Eckhardt, S. G., Messersmith, W. A. & Jimeno, A. The development of regorafenib and its current and potential future role in cancer therapy. Drugs Today (Barc) 49, 105–115 (2013).
Hudkins, R. L. et al. Synthesis and biological profile of the pan-vascular endothelial growth factor receptor/tyrosine kinase with immunoglobulin and epidermal growth factor-like homology domains 2 (VEGF-R/TIE-2) inhibitor 11-(2-methylpropyl)-12,13-dihydro-2-methyl-8-(pyrimidin-2-ylamino)-4H-indazolo[5, 4-a]pyrrolo[3,4-c]carbazol-4-one: a novel oncology therapeutic agent. J. Med. Chem. 55, 903–913 (2012).
Karnezis, T., Shayan, R., Fox, S., Achen, M. G. & Stacker, S. A. The connection between lymphangiogenic signalling and prostaglandin biology: a missing link in the metastatic pathway. Oncotarget 3, 893–906 (2012).
Joukov, V. et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 290–298 (1996); erratum 15, 1751 (1996).
Achen, M. G. et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl Acad. Sci. USA 95, 548–553 (1998). References 198 and 199 describe the characterization of VEGFC and VEGFD and their relationship to the VEGF family, as well as their proteolytic cleavage and activity on endothelial cells.
Qian, C. N. et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 66, 10365–10376 (2006). References 69 and 200 highlight that primary tumours might prepare future sites of metastasis, such as lymph nodes, by releasing lymphangiogenic growth factors.
Acknowledgements
S.A.S., T.K. and M.G.A. are supported by Project Grants, a Program Grant and Research Fellowships from the National Health and Medical Research Council (NHMRC) of Australia, and by funds from the Operational Infrastructure Support Program that is provided by the Victorian Government, Australia. R.S. has been supported by the Raelene Boyle Sporting Chance Cancer Foundation and the Royal Australasian College of Surgeons (RACS) Foundation Scholarship, as well as the RACS Surgeon Scientist Program. S.P.W. has been supported by an Australian National Breast Cancer Foundation Doctoral Research Fellowship. The authors thank M. Macheda for proofreading.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
S.A.S. and M.G.A. have both been consultants for Vegenics Limited, South Yarra, Victoria, Australia, in the area of developing inhibitors of angiogenesis and lymphangiogenesis in human diseases, including cancer. This consultancy ended on 31 December 2012. S.A.S., R.S. and M.G.A. are shareholders in Circadian Technologies (owner of Vegenics), South Yarra, Victoria, Australia, which is a company that develops anticancer therapeutics, and in Ark Therapeutics Group Plc, London, UK, which has been developing therapeutic approaches based on the vascular endothelial growth factor family. S.A.S., S.B.F. and M.G.A. are holders of an Australian National Health and Medical Research Council (NHMRC) Program Grant. S.A.S. and M.G.A. are holders of NHMRC Senior Research Fellowships in this area. S.A.S., S.B.F., T.K. and M.G.A. are holders of NHMRC Project Grants in this area. S.A.S. and M.G.A. are holders of numerous patents in this area. S.P.W. declares no competing interests.
Glossary
- Lymphangiogenesis
-
The formation of new lymphatic vessels from pre-existing lymphatics.
- Lymphatic enlargement
-
The enlargement of lymphatics, which can occur via proliferation of lymphatic endothelial cells (that is, hyperplasia) or other non-proliferative mechanisms.
- Lymphatic hyperplasia
-
The enlargement of lymphatics by proliferation of lymphatic endothelial cells, which may or may not be accompanied by sprouting.
- Initial lymphatics
-
Small blind-ended lymphatics in the tissue periphery that are adapted for the uptake of fluid and cells.
- Collecting lymphatics
-
Large lymphatics that are characterized by a continuous smooth muscle cell coating and are adapted for the transport of lymph and associated cells.
- Lymphatic remodelling in cancer
-
Alteration to the structure and morphology of lymphatic vessels that are associated with cancer, including lymphangiogenesis and lymphatic enlargement.
- Lymphatic invasion
-
The entry of tumour cells into lymphatics, which is identified clinicopathologically by the detection of tumour cells or tumour cell emboli within lymphatics.
Rights and permissions
About this article
Cite this article
Stacker, S., Williams, S., Karnezis, T. et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14, 159–172 (2014). https://doi.org/10.1038/nrc3677
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3677
This article is cited by
-
Anti-lymphangiogenesis for boosting drug accumulation in tumors
Signal Transduction and Targeted Therapy (2024)
-
The role of HIF in angiogenesis, lymphangiogenesis, and tumor microenvironment in urological cancers
Molecular Biology Reports (2024)
-
Lymphangiogenesis in gastric cancer: function and mechanism
European Journal of Medical Research (2023)
-
Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells
Journal of Experimental & Clinical Cancer Research (2023)
-
Loss of TTC17 promotes breast cancer metastasis through RAP1/CDC42 signaling and sensitizes it to rapamycin and paclitaxel
Cell & Bioscience (2023)