Key Points
-
Hypoxia represents a compelling therapeutic target, given that it has a major role in tumour development and resistance to therapy, and that the levels of hypoxia are more severe in most tumours than normal tissues.
-
One approach to targeting hypoxia seeks to develop bioreductive prodrugs that are activated by enzymatic reduction in hypoxic tissue. These prodrugs are chemically diverse and represent two distinct strategies: activation under moderate hypoxia (as exemplified by tirapazamine) or only under severe hypoxia (as exemplified by PR-104). In the latter case, diffusion of the active drug to less hypoxic cells is essential.
-
A second approach seeks small molecule inhibitors against molecular targets involved in the survival of hypoxic cells. Current interest focuses on the inhibition of the hypoxia-inducible factor 1 (HIF1), the unfolded protein response (UPR) and mTOR pathways, but the most important vulnerabilities in hypoxic cells are not well defined. Most molecularly targeted agents have been 'repurposed' from other applications, and have low selectivity as hypoxic cytotoxins.
-
Both approaches face substantial challenges in relation to off-target effects, which, ironically, also present opportunities. For bioreductive prodrugs, activation by aerobic reductases can contribute to normal tissue toxicity, but this is exploitable in tumours that highly express these enzymes. For molecularly targeted agents, hypoxia-independent signalling through the same pathways may provide opportunities for additional antitumour activity.
-
Both bioreductive prodrugs and molecularly targeted agents also need to overcome the problem of drug penetration through poorly perfused hypoxic tissue; strategies for addressing this requirement are being developed.
-
The current generation of bioreductive prodrugs generate DNA-reactive cytotoxins, making them difficult to combine with conventional chemotherapy because of overlapping toxicity. This challenge is stimulating the development of bioreductive prodrugs that release molecularly targeted agents as their effectors, potentially combining the best features of both approaches.
-
Given the marked heterogeneity in hypoxia between tumours of the same type, the clinical exploitation of hypoxia using all of these approaches will require their co-development with companion diagnostics for hypoxia (and for other determinants of sensitivity).
Abstract
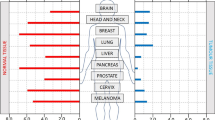
Hypoxia is a feature of most tumours, albeit with variable incidence and severity within a given patient population. It is a negative prognostic and predictive factor owing to its multiple contributions to chemoresistance, radioresistance, angiogenesis, vasculogenesis, invasiveness, metastasis, resistance to cell death, altered metabolism and genomic instability. Given its central role in tumour progression and resistance to therapy, tumour hypoxia might well be considered the best validated target that has yet to be exploited in oncology. However, despite an explosion of information on hypoxia, there are still major questions to be addressed if the long-standing goal of exploiting tumour hypoxia is to be realized. Here, we review the two main approaches, namely bioreductive prodrugs and inhibitors of molecular targets upon which hypoxic cell survival depends. We address the particular challenges and opportunities these overlapping strategies present, and discuss the central importance of emerging diagnostic tools for patient stratification in targeting hypoxia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Pries, A. R. et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput. Biol. 5, e1000394 (2009).
Dewhirst, M. W., Cao, Y. & Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature Rev. Cancer 8, 425–437 (2008).
Graeber, T. G. et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379, 88–91 (1996).
Erler, J. T. et al. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol. Cell. Biol. 24, 2875–2889 (2004).
Rouschop, K. M. et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Invest. 120, 127–141 (2010). This study demonstrates a mechanism by which the UPR enhances the survival of tumour cells under severe hypoxia and that inhibition of the UPR by a small molecule (chloroquine) selectively kills hypoxic cells.
Cairns, R. A., Harris, I. S. & Mak, T. W. Regulation of cancer cell metabolism. Nature Rev. Cancer 11, 85–95 (2011).
Wang, Y. & Ohh, M. Oxygen-mediated endocytosis in cancer. J. Cell. Mol. Med. 14, 496–503 (2010).
Semenza, G. L. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit. Rev. Biochem. Mol. Biol. 35, 71–103 (2000).
Kioi, M. et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 120, 694–705 (2010).
Hill, R. P., Marie-Egyptienne, D. T. & Hedley, D. W. Cancer stem cells, hypoxia and metastasis. Semin. Radiat. Oncol. 19, 106–111 (2009).
Pennacchietti, S. et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 3, 347–361 (2003).
Chang, Q., Jurisica, I., Do, T. & Hedley, D. W. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 78, 3110–3120 (2011).
Yotnda, P., Wu, D. & Swanson, A. M. Hypoxic tumours and their effect on immune cells and cancer therapy. Methods Mol. Biol. 651, 1–29 (2010).
Guzy, R. D. et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell. Metab. 1, 401–408 (2005).
Bristow, R. G. & Hill, R. P. Hypoxia, DNA repair and genetic instability. Nature Rev. Cancer 8, 180–192 (2008).
Vaupel, P., Hockel, M. & Mayer, A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid. Redox Signal. 9, 1221–1235 (2007).
Tatum, J. L. et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol. 82, 699–757 (2006).
Jubb, A. M., Buffa, F. M. & Harris, A. L. Assessment of tumour hypoxia for prediction of response to therapy and cancer prognosis. J. Cell. Mol. Med. 14, 18–29 (2010).
Connors, T. A. & Whisson, M. E. Cure of mice bearing advanced plasma cell tumours with aniline mustard: the relationship between glucuronidase activity and tumour sensitivity. Nature 210, 866–867 (1966).
Mason, R. P. & Holtzman, J. L. The role of catalytic superoxide formation in the O2 inhibition of nitroreductase. Biochem. Biophys. Res. Commun. 67, 1267–1274 (1975). The discovery of the mechanism by which oxygen inhibits the reduction of substrates by one-electron reductases.
Mohindra, J. K. & Rauth, A. M. Increased cell killing by metronidazole and nitrofurazone of hypoxic compared to aerobic mammalian cells. Cancer Res. 36, 930–936 (1976).
Adams, G. E., Dische, S., Fowler, J. F. & Thomlinson, R. H. Hypoxic cell sensitisers in radiotherapy. Lancet 1, 186–188 (1976).
Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin. Oncol. 19, 397–417 (2007).
Schwartz, H. S., Sodergren, J. E. & Philips, F. S. Mitomycin C: chemical and biological studies on alkylation. Science 142, 1181–1183 (1963).
Iyer, V. N. & Szybalski, W. Mitomycins and porfiromycins: chemical mechanism of activation and cross-linking of DNA. Science 145, 55–58 (1964).
Lin, A. J., Cosby, L. A., Shanky, C. W. & Sartorelli, A. C. Potential bioreductive alkylating agents. I. Benzoquinone derivatives. J. Med. Chem. 15, 1247–1252 (1972).
Carter, D. B. & Phillips, A. F. Measurement of electrode potentials in living and dead tissues. Nature 174, 121–123 (1954).
Kennedy, K. A., Rockwell, S. & Sartorelli, A. C. Preferential activation of mitomycin C to cytotoxic metabolites by hypoxic tumor cells. Cancer Res. 40, 2356–2360 (1980).
Bachur, N. R., Gordon, S. L. & Gee, M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 38, 1745–1750 (1978).
Brown, J. M. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br. J. Cancer 67, 1163–1170 (1993).
Chowdhury, G., Junnotula, V., Daniels, J. S., Greenberg, M. M. & Gates, K. S. DNA strand damage product analysis provides evidence that the tumor cell-specific cytotoxin tirapazamine produces hydroxyl radical and acts as a surrogate for O2 . J. Am. Chem. Soc. 129, 12870–12877 (2007).
Shinde, S. S., Hay, M. P., Patterson, A. V., Denny, W. A. & Anderson, R. F. Spin trapping of radicals other than the *OH radical upon reduction of the anticancer agent tirapazamine by cytochrome P450 reductase. J. Am. Chem. Soc. 131, 14220–14221 (2009).
Patterson, L. H. Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metastasis Rev. 12, 119–134 (1993).
Wilson, W. R., van Zijl, P. & Denny, W. A. Bis-bioreductive agents as hypoxia-selective cytotoxins: nitracrine N-oxide. Int. J. Radiat. Oncol. Biol. Phys. 22, 693–696 (1992).
Ware, D. C., Palmer, B. D., Wilson, W. R. & Denny, W. A. Hypoxia-selective antitumor agents. 7. Metal complexes of aliphatic mustards as a new class of hypoxia-selective cytotoxins. Synthesis and evaluation of cobalt(III) complexes of bidentate mustards. J. Med. Chem. 36, 1839–1846 (1993). The first use of transitional metal complexes as the basis for the design of hypoxia-activated prodrugs.
Ahn, G. O. et al. Radiolytic and cellular reduction of a novel hypoxia-activated cobalt(III) prodrug of a chloromethylbenzindoline DNA minor groove alkylator. Biochem. Pharmacol. 71, 1683–1694 (2006).
Parker, L. L. et al. A novel design strategy for stable metal complexes of nitrogen mustards as bioreductive prodrugs. J. Med. Chem. 47, 5683–5689 (2004).
Weiss, G. J. et al. Phase 1 study of the safety, tolerability and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin. Cancer Res. 17, 2997–3004 (2011). The first clinical data for this novel hypoxia-targeted prodrug, showing evidence of tumour responses when used as a monotherapy.
Wardman, P. Electron transfer and oxidative stress as key factors in the design of drugs selectively active in hypoxia. Curr. Med. Chem. 8, 739–761 (2001).
Stratford, I. J., Williams, K. J., Cowen, R. L. & Jaffar, M. Combining bioreductive drugs and radiation for the treatment of solid tumors. Semin. Radiat. Oncol. 13, 42–52 (2003).
Ahn, G. O. & Brown, M. Targeting tumors with hypoxia-activated cytotoxins. Front. Biosci. 12, 3483–3501 (2007).
Chen, Y. & Hu, L. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 29, 29–64 (2009).
Tercel, M. et al. Selective treatment of hypoxic tumor cells in vivo: phosphate pre-prodrugs of nitro analogues of the duocarmycins. Angew. Chem. Int. Ed. 50, 2606–2609 (2011). A report of a novel class of hypoxia-activated prodrugs of DNA minor groove alkylators showing potent and selective killing of hypoxic cells in xenograft models.
Evans, J. W. et al. Homologous recombination is the principal pathway for the repair of DNA damage induced by tirapazamine in mammalian cells. Cancer Res. 68, 257–265 (2008). Evidence for the critical importance of HR-mediated DNA repair in determining the sensitivity of hypoxic cells to TPZ.
Gu, Y. et al. Roles of DNA repair and reductase activity in the cytotoxicity of the hypoxia-activated dinitrobenzamide mustard PR-104A. Mol. Cancer Ther. 8, 1714–1723 (2009).
Branzei, D. & Foiani, M. Maintaining genome stability at the replication fork. Nature Rev. Mol. Cell Biol. 11, 208–219 (2010).
Raleigh, S. M., Wanogho, E., Burke, M. D., McKeown, S. R. & Patterson, L. H. Involvement of human cytochromes P450 (CYP) in the reductive metabolism of AQ4N, a hypoxia activated anthraquinone di-N-oxide prodrug. Int. J. Radiat. Oncol. Biol. Phys. 42, 763–767 (1998).
Nishida, C. R., Lee, M. & Ortiz de Montellano, P. R. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol. Pharmacol. 78, 497–502 (2010). A demonstration that the 'orphan' cytochrome P450 CYP2S1, which is upregulated under hypoxia, catalyses hypoxic activation of the bioreductive prodrug AQ4N.
Rivera, S. P. et al. A novel promoter element containing multiple overlapping xenobiotic and hypoxia response elements mediates induction of cytochrome P4502S1 by both dioxin and hypoxia. J. Biol. Chem. 282, 10881–10893 (2007).
Nishida, C. R. & Ortiz de Montellano, P. R. Reductive heme-dependent activation of the N-oxide prodrug AQ4N by nitric oxide synthase. J. Med. Chem. 51, 5118–5120 (2008).
Colucci, M. A., Moody, C. J. & Couch, G. D. Natural and synthetic quinones and their reduction by the quinone reductase enzyme NQO1: from synthetic organic chemistry to compounds with anticancer potential. Org. Biomol. Chem. 6, 637–656 (2008).
Knox, R. J. et al. The nitroreductase enzyme in Walker cells that activates 5-(aziridin-1-yl)-2,4-dinitro-benzamide (CB 1954) to 5-(aziridin-1-yl)-4-hydroxyla-mino-2-nitrobenzamide is a form of NAD(P)H dehydrogenase (quinone) (EC 1.6.99.2). Biochem. Pharmacol. 37, 4671–4677 (1988). The first characterization of an oxygen-independent two-electron reductase responsible for the activation of a bioreductive prodrug.
Celli, C. M., Tran, N., Knox, R. & Jaiswal, A. K. NRH:quinone oxidoreductase 2 (NQO2) catalyzes metabolic activation of quinones and anti-tumor drugs. Biochem. Pharmacol. 72, 366–376 (2006).
Yan, C., Kepa, J. K., Siegel, D., Stratford, I. J. & Ross, D. Dissecting the role of multiple reductases in bioactivation and cytotoxicity of the antitumor agent 2, 5-diaziridinyl-3-(hydroxymethyl)-6-methyl-1,4-benzoquinone (RH1). Mol. Pharmacol. 74, 1657–1665 (2008).
Guise, C. P. et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 70, 1573–1584 (2010). The surprising observation that AKR1C3, which is highly expressed in some tumours, activates PR-104A (but not other bioreductive prodrugs) by a two-electron reduction.
Adikesavan, A. K., Barrios, R. & Jaiswal, A. K. In vivo role of NAD(P)H:quinone oxidoreductase 1 in metabolic activation of mitomycin C and bone marrow cytotoxicity. Cancer Res. 67, 7966–7971 (2007).
Siegel, D. & Ross, D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues 6. Free Rad. Biol. Med. 29, 246–253 (2000).
Wang, W. & Jaiswal, A. K. Nuclear factor Nrf2 and antioxidant response element regulate NRH:quinone oxidoreductase 2 (NQO2) gene expression and antioxidant induction. Free Rad. Biol. Med. 40, 1119–1130 (2006).
MacLeod, A. K. et al. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis 30, 1571–1580 (2009).
Cullinan, S. B. & Diehl, J. A. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell. Biol. 38, 317–332 (2006).
Jain, A. et al. Response of multiple recurrent TaT1 bladder cancer to intravesical apaziquone (EO9): comparative analysis of tumor recurrence rates. Urology 73, 1083–1086 (2009).
Danson, S. J. et al. Phase I pharmacokinetic and pharmacodynamic study of the bioreductive drug RH1. Ann. Oncol. 4 Mar 2011 (doi:10.1093/annonc/mdq638).
Birtwistle, J. et al. The aldo-keto reductase AKR1C3 contributes to 7,12-dimethylbenz(a)anthracene-3,4-dihydrodiol mediated oxidative DNA damage in myeloid cells: implications for leukemogenesis. Mutat. Res. 662, 67–74 (2009).
Konopleva, M. et al. Therapeutic targeting of the hypoxic microenvironment in acute lymphocytic leukemia. Blood (ASH Annual Meeting Abstracts) 114, Abstract 2040 [online] (2009).
Hu, J. et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood 116, 1524–1527 (2010).
Baker, M. A., Zeman, E. M., Hirst, V. K. & Brown, J. M. Metabolism of SR 4233 by Chinese hamster ovary cells: basis of selective hypoxic cytotoxicity. Cancer Res. 48, 5947–5952 (1988).
Hicks, K. O. et al. Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin. Cancer Res. 16, 4946–4957 (2010). The development of a second-generation benzotriazine dioxide analogue of TPZ using a novel testing algorithm based on spatially resolved pharmacokinetic and pharmacodynamic modelling.
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nature Rev. Cancer 6, 583–592 (2006).
Durand, R. E. & Olive, P. L. Physiologic and cytotoxic effects of tirapazamine in tumor-bearing mice. Radiat. Oncol. Investig. 5, 213–219 (1997).
Durand, R. E. & Olive, P. L. Evaluation of bioreductive drugs in multicell spheroids. Int. J. Radiat. Oncol. Biol. Phys. 22, 689–692 (1992).
Hicks, K. O., Fleming, Y., Siim, B. G., Koch, C. J. & Wilson, W. R. Extravascular diffusion of tirapazamine: effect of metabolic consumption assessed using the multicellular layer model. Int. J. Radiat. Oncol. Biol. Phys. 42, 641–649 (1998).
Kyle, A. H. & Minchinton, A. I. Measurement of delivery and metabolism of tirapazamine to tumour tissue using the multilayered cell culture model. Cancer Chemother. Pharmacol. 43, 213–220 (1999).
Hicks, K. O. et al. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J. Natl Cancer Inst. 98, 1118–1128 (2006). Conclusive evidence that hypoxic cell killing by TPZ and its analogues, in xenografts, is limited by the ability of these drugs to penetrate hypoxic tumour tissue.
Pruijn, F. B., Patel, K., Hay, M. P., Wilson, W. R. & Hicks, K. O. Prediction of tumour tissue diffusion coefficients of hypoxia-activated prodrugs from physicochemical parameters. Aust. J. Chem. 61, 687–693 (2008).
Hay, M. P. et al. Hypoxia-selective 3-alkyl 1,2,4-benzotriazine 1,4-dioxides: the influence of hydrogen bond donors on extravascular transport and antitumor activity. J. Med. Chem. 50, 6654–6664 (2007).
Koch, C. J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 53, 3992–3997 (1993). The first report that the inhibition of TPZ cytotoxicity requires higher oxygen concentrations than for nitro compounds and quinones.
Hicks, K. O., Siim, B. G., Pruijn, F. B. & Wilson, W. R. Oxygen dependence of the metabolic activation and cytotoxicity of tirapazamine: implications for extravascular transport and activity in tumors. Radiat. Res. 161, 656–666 (2004).
Marshall, R. S. & Rauth, A. M. Oxygen and exposure kinetics as factors influencing the cytotoxicity of porfiromycin, a mitomycin C analogue, in Chinese hamster ovary cells. Cancer Res. 48, 5655–5659 (1988).
Siim, B. G., Atwell, G. J. & Wilson, W. R. Oxygen dependence of the cytotoxicity and metabolic activation of 4-alkylamino-5-nitroquinoline bioreductive drugs. Br. J. Cancer 70, 596–603 (1994).
Wilson, W. R., Moselen, J. W., Cliffe, S., Denny, W. A. & Ware, D. C. Exploiting tumor hypoxia through bioreductive release of diffusible cytotoxins: the cobalt(III)-nitrogen mustard complex SN 24771. Int. J. Radiat. Oncol. Biol. Phys. 29, 323–327 (1994).
Wilson, W. R. et al. Bystander effects of bioreductive drugs: potential for exploiting pathological tumor hypoxia with dinitrobenzamide mustards. Radiat. Res. 167, 625–636 (2007).
Hicks, K. O. et al. Oxygen dependence and extravascular transport of hypoxia-activated prodrugs: comparison of the dinitrobenzamide mustard PR-104A and tirapazamine. Int. J. Radiat. Oncol. Biol. Phys. 69, 560–571 (2007).
Helleday, T., Petermann, E., Lundin, C., Hodgson, B. & Sharma, R. A. DNA repair pathways as targets for cancer therapy. Nature Rev. Cancer 8, 193–204 (2008).
Parveen, I., Naughton, D. P., Whish, W. J. & Threadgill, M. D. 2-nitroimidazol-5-ylmethyl as a potential bioreductively activated prodrug system: reductively triggered release of the PARP inhibitor 5-bromoisoquinolinone. Bioorg. Med. Chem. Lett. 9, 2031–2036 (1999).
Everett, S. A., Naylor, M. A., Patel, K. B., Stratford, M. R. L. & Wardman, P. Bioreductively-activated prodrugs for targetting hypoxic tissues: elimination of aspirin from 2-nitroimidazole derivatives. Bioorg. Med. Chem. Lett. 9, 1267–1272 (1999).
Thomson, P. et al. Synthesis and biological properties of bioreductively targeted nitrothienyl prodrugs of combretastatin A-4. Mol. Cancer Ther. 5, 2886–2894 (2006).
Granchi, C. et al. Bioreductively activated lysyl oxidase inhibitors against hypoxic tumours. ChemMedChem 4, 1590–1594 (2009).
Tercel, M., Wilson, W. R., Anderson, R. F. & Denny, W. A. Hypoxia-selective antitumor agents. 12. Nitrobenzyl quaternary salts as bioreductive prodrugs of the alkylating agent mechlorethamine. J. Med. Chem. 39, 1084–1094 (1996).
Patterson, A. V. et al. Cellular metabolism, murine pharmacokinetics and preclinical antitumor activity of SN29966, a novel hypoxia-activated irreversible pan-HER inhibitor. Mol. Cancer Ther. 8 (Suppl. 1), B76 (2009).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Poon, E., Harris, A. L. & Ashcroft, M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev. Mol. Med. 11, e26 (2009).
Wouters, B. G. & Koritzinsky, M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nature Rev. Cancer. 8, 851–864 (2008).
Martinive, P. et al. Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: implications for cancer therapies. Cancer Res. 66, 11736–11744 (2006).
Semenza, G. L. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today 12, 853–859 (2007).
Giaccia, A., Siim, B. G. & Johnson, R. S. HIF-1 as a target for drug development. Nature Rev. Drug Discov. 2, 803–811 (2003).
Melillo, G. Targeting hypoxia cell signalling for cancer therapy. Cancer Metastasis Rev. 26, 341–352 (2007).
Isaacs, J. S. et al. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J. Biol. Chem. 277, 29936–29944 (2002).
Kizaka-Kondoh, S., Tanaka, S., Harada, H. & Hiraoka, M. The HIF-1-active microenvironment: an environmental target for cancer therapy. Advanced Drug Deliv. Rev. 61, 623–632 (2009).
Rouschop, K. M. & Wouters, B. G. Regulation of autophagy through multiple independent hypoxic signaling pathways. Curr. Mol. Med. 9, 417–424 (2009).
Tu, B. P. & Weissman, J. S. The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 10, 983–994 (2002).
Koditz, J. et al. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood 110, 3610–3617 (2007).
Bi, M. et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 (2005).
Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004). The identification of IRE1 as a target for killing hypoxic cells.
Spiotto, M. T. et al. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer Res. 70, 78–88 (2010).
Papandreou, I. et al. Identification of an Ire1α endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 117, 1311–1314 (2011).
Volkmann, K. et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J. Biol. Chem. 286, 12743–12755 (2011). The first demonstration of salicaldehydes as novel IRE1 inhibitors in vitro and in vivo.
Lee, K. P. et al. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 132, 89–100 (2008).
Fels, D. R. et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res. 68, 9323–9330 (2008).
Koumenis, C. & Wouters, B. G. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol. Cancer Res. 4, 423–436 (2006).
Brugarolas, J. et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 (2004).
Liu, L. et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21, 521–531 (2006).
Li, Y. et al. BNIP3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J. Biol. Chem. 282, 35803–35813 (2007).
Pencreach, E. et al. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia-inducible factor-1α axis. Clin. Cancer Res. 15, 1297–1307 (2009).
Yu, K. et al. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 70, 621–631 (2010).
Rzymski, T. et al. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene 29, 4424–4435 (2010).
Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S. & Moreno-Sanchez, R. HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini-Rev. Med. Chem. 9, 1084–1101 (2009).
Levine, A. J. & Puzio-Kuter, A. M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330, 1340–1344 (2010).
Kurtoglu, M., Maher, J. C. & Lampidis, T. J. Differential toxic mechanisms of 2-deoxy-D-glucose versus 2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells. Antiox. Redox Signal. 9, 1383–1390 (2007). A critical review addressing the mechanism of hypoxia-selective cytotoxicity of glucose analogues.
Song, C. W., Clement, J. J. & Levitt, S. H. Preferential cytotoxicity of 5-thio-D-glucose against hypoxic tumor cells. J. Natl Cancer Inst. 57, 603–605 (1976).
Lampidis, T. J. et al. Efficacy of 2-halogen substituted D-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”. Cancer Chemother. Pharmacol. 58, 725–734 (2006).
Macheda, M. L., Rogers, S. & Best, J. D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell. Physiol. 202, 654–662 (2005).
Wood, T. E. et al. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol. Cancer Ther. 7, 3546–3555 (2008).
Lai, E. W., Chan, D. A., Hay, M. P. & Giaccia, A. J. Selective cytotoxic targeting of von Hippel-Lindau-deficient renal carcinoma cells. Proc. Am. Assoc. Cancer Res. 51, Abstract 67 (2010).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 (2008). A demonstration that inhibition of MCT1 blocks the use of lactate for respiration in aerobic cells, eliciting rapid glucose consumption and death of hypoxic cells owing to glucose starvation.
Ovens, M. J., Davies, A. J., Wilson, M. C., Murray, C. M. & Halestrap, A. P. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem. J. 425, 523–530 (2010).
Murray, C. M. et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nature Chem. Biol. 1, 371–376 (2005).
Chiche, J., Brahimi-Horn, M. C. & Pouyssegur, J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J. Cell. Mol. Med. 14, 771–794 (2010).
Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 281, 9030–9037 (2006).
Gallagher, S. M., Castorino, J. J., Wang, D. & Philp, N. J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 67, 4182–4189 (2007).
Chiche, J. et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 69, 358–368 (2009).
van den Beucken, T. et al. Hypoxia-induced expression of carbonic anhydrase 9 is dependent on the unfolded protein response. J. Biol. Chem. 284, 24204–24212 (2009).
Lou, Y. et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 71, 3364–3376 (2011).
Huang, L. E., Bindra, R. S., Glazer, P. M. & Harris, A. L. Hypoxia-induced genetic instability-a calculated mechanism underlying tumor progression. J. Mol. Med. 85, 139–148 (2007).
Olcina, M., Lecane, P. S. & Hammond, E. M. Targeting hypoxic cells through the DNA damage response. Clin. Cancer Res. 16, 5620–5629 (2010).
Chan, N., Koch, C. J. & Bristow, R. G. Tumor hypoxia as a modifier of DNA strand break and cross-link repair. Curr. Mol. Med. 9, 401–410 (2009).
Hammond, E. M., Green, S. L. & Giaccia, A. J. Comparison of hypoxia-induced replication arrest with hydroxyurea and aphidicolin-induced arrest. Mutat. Res. 532, 205–213 (2003).
Pires, I. M. et al. Effects of acute versus chronic hypoxia on DNA damage responses and genomic instability. Cancer Res. 70, 925–935 (2010). A demonstration that severe hypoxia induces DNA replication arrest through loss of ribonucleotide reductase activity and suppression of dNTP pools.
Norlund, P. & Reichard, P. Ribonucleotide reductases. Ann. Rev. Biochem. 75, 681–706 (2006).
Hammond, E. M., Dorie, M. J. & Giaccia, A. J. Inhibition of ATR leads to increased sensitivity to hypoxia/reoxygenation. Cancer Res. 64, 6556–6562 (2004).
Hammond, E. M., Dorie, M. J. & Giaccia, A. J. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J. Biol. Chem. 278, 12207–12213 (2003).
Gibson, S. L., Bindra, R. S. & Glazer, P. M. CHK2-dependent phosphorylation of BRCA1 in hypoxia. Radiat. Res. 166, 646–651 (2006).
Chang, C.-J. et al. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-β-catenin signaling. Cancer Cell 19, 86–100 (2011).
Marotta, D. et al. In vivo profiling of hypoxic gene expression in gliomas using the hypoxia marker EF5 and laser-capture microdissection. Cancer Res. 71, 779–789 (2011).
Bindra, R. S. et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol. Cell. Biol. 24, 8504–8518 (2004). An early demonstration of the effects of hypoxia on HR repair and the implications of hypoxia for genomic instability and therapy.
Chan, N. et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 68, 605–614 (2008). A demonstration of selective killing of hypoxic cells by a PARP inhibitor in cell culture and in xenografts.
Chan, N. et al. Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res. 70, 8045–8054 (2010).
Di Cintio, A., Di Gennaro, E. & Budillon, A. Restoring p53 function in cancer: novel therapeutic approaches for applying the brakes to tumorigenesis. Recent Pat. Anti-Cancer Drug Discov. 5, 1–13 (2010).
Yang, J. et al. Small-molecule activation of p53 blocks hypoxia-inducible factor 1α and vascular endothelial growth factor expression in vivo and leads to tumor cell apoptosis in normoxia and hypoxia. Mol. Cell. Biol. 29, 2243–2253 (2009).
Hoogsteen, I. J. et al. Hypoxia in larynx carcinomas assessed by pimonidazole binding and the value of CA-IX and vascularity as surrogate markers of hypoxia. Eur. J. Cancer 45, 2906–2914 (2009).
Nordsmark, M. et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 77, 18–24 (2005). A meta-analysis of the prognostic significance of tumour hypoxia in head and neck tumours, assessed with oxygen electrodes, demonstrating a major impact on outcome after radiotherapy.
Mees, G., Dierckx, R., Vangestel, C. & Van De, W. C. Molecular imaging of hypoxia with radiolabelled agents. Eur. J. Nucl. Med. Mol. Imag. 36, 1674–1686 (2009).
Mason, R. P. et al. Multimodality imaging of hypoxia in preclinical settings. Quart. J. Nucl. Med. Mol. Imag. 54, 259–280 (2010).
Workman, P. & Stratford, I. J. The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev. 12, 73–82 (1993).
Willers, H. et al. Utility of DNA repair protein foci for the detection of putative BRCA1 pathway defects in breast cancer biopsies. Mol. Cancer Res. 7, 1304–1309 (2009).
Konstantinopoulos, P. A. et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J. Clin. Oncol. 28, 3555–3561 (2010).
Graeser, M. et al. A marker of homologous recombination predicts pathological complete response to neoadjuvant chemotherapy in primary breast cancer. Clin. Cancer Res. 16, 6159–6158 (2010).
Wang, J. et al. EF5 as a predictive biomarker for activation of the new hypoxia targeting prodrug SN30000. Am. Soc. Clin. Oncol. Abstract e13597, (2011).
Hedley, D. et al. Carbonic anhydrase IX expression, hypoxia, and prognosis in patients with uterine cervical carcinomas. Clin. Cancer Res. 9, 5666–5674 (2003).
Whitehurst, A. W. et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature 446, 815–819 (2007).
Barabasi, A.-L., Gulbache, N. & Loscalzo, J. Network medicine: a network-based approach to human disease. Nature Rev. Genet. 12, 56–68 (2011).
Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5, 429–441 (2004).
Garner, A. P. et al. Nitric oxide synthases catalyze the activation of redox cycling and bioreductive anticancer agents. Cancer Res. 59, 1929–1934 (1999).
Chinje, E. C. et al. Non-nuclear localized human NOSII enhances the bioactivation and toxicity of tirapazamine (SR4233) in vitro. Mol. Pharmacol. 63, 1248–1255 (2003).
Thomsen, L. L. et al. Nitric oxide synthase activity in human breast cancer. Br. J. Cancer 72, 41–44 (1995).
Swana, H. S. et al. Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. J. Urol. 161, 630–634 (1999).
Lewis, C. & Murdoch, C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am. J. Pathol. 167, 627–635 (2005).
Melillo, G. et al. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J. Exp. Med. 182, 1683–1693 (1995).
Tendler, D. S. et al. Intersection of interferon and hypoxia signal transduction pathways in nitric oxide-induced tumor apoptosis. Cancer Res. 61, 3682–3688 (2001).
Chinje, E. C. et al. 17β-Oestradiol treatment modulates nitric oxide synthase activity in MDA231 tumour with implications on growth and radiation response. Br. J. Cancer 86, 136–142 (2002).
Patterson, L. H. & McKeown, S. R. AQ4N: a new approach to hypoxia-activated cancer chemotherapy. Br. J. Cancer 83, 1589–1593 (2000).
Chandor, A. et al. Metabolic activation of the antitumor drug 5-(Aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) by NO synthases. Chem. Res. Toxicol. 21, 836–843 (2008).
Guise, C. P. et al. Identification of human oxidoreductases involved in the hypoxia-dependent activation of bioreductive prodrugs. Proc. Am. Assoc. Cancer Res. 51, Abstract 453 (2010).
Kan, O. et al. Genetically modified macrophages expressing hypoxia regulated cytochrome P450 and P450 reductase for the treatment of cancer. Int. J. Mol. Med. 27, 173–180 (2011).
Cowen, R. L. et al. Hypoxia targeted gene therapy to increase the efficacy of tirapazamine as an adjuvant to radiotherapy: reversing tumor radioresistance and effecting cure. Cancer Res. 64, 1396–1402 (2004).
Hodnick, W. F. & Sartorelli, A. C. Reductive activation of mitomycin C by NADH:cytochrome b5 reductase. Cancer Res. 53, 4907–4912 (1993).
Miskiniene, V., Dickancaite, E., Nemeikaite, A. & Cenas, N. Nitroaromatic betulin derivatives as redox cycling agents. Biochem. Mol. Biol. Int. 42, 391–397 (1997).
Kutcher, W. W. & McCalla, D. R. Aerobic reduction of 5-nitro-2-furaldehyde semicarbazone by rat liver xanthine dehydrogenase. Biochem. Pharmacol. 33, 799–805 (1984).
Ahlskog, J. K. et al. Human monoclonal antibodies targeting carbonic anhydrase IX for the molecular imaging of hypoxic regions in solid tumours. Br. J. Cancer 101, 645–657 (2009).
Hoeben, B. A. et al. PET of hypoxia with 89Zr-labeled cG250-F(ab')2 in head and neck tumors. J. Nucl. Med. 51, 1076–1083 (2010).
Kaluz, S., Kaluzova, M., Liao, S. Y., Lerman, M. & Stanbridge, E. J. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: a one transcription factor (HIF-1) show? Biochim. Biophys. Acta 1795, 162–172 (2009).
Komar, G. et al. 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J. Nucl. Med. 49, 1944–1951 (2008).
Rischin, D. et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J. Clin. Oncol. 24, 2098–2104 (2006). A provocative study, from a single clinical centre, demonstrating that TPZ-responsive patients can be identified by PET imaging of hypoxia.
Rischin, D. et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J. Clin. Oncol. 28, 2989–2995 (2010).
Wouters, B. G. & Brown, J. M. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat. Res. 147, 541–550 (1997). An important theoretical study that makes the case that moderately hypoxic (partially radioresistant) cells are more important to radiotherapy outcome than the most hypoxic cells in tumours.
Tuttle, S. W. et al. Detection of reactive oxygen species via endogenous oxidative pentose phosphate cycle activity in response to oxygen concentration. J. Biol. Chem. 282, 36790–36796 (2007).
Rzymski, T. & Harris, A. L. The unfolded protein response and integrated stress response to anoxia. Clin. Cancer Res. 13, 2537–2540 (2007).
Acknowledgements
We thank our collaborators (R. Anderson, W. Denny, K. Hicks, C. Guise, A. Patterson, F. Pruijn, J. Smaill, M. Tercel and J. Wang) for many fruitful discussions that have helped to inform the views expressed here. The authors acknowledge financial support from the Health Research Council of New Zealand (W.R.W.) and the Maurice Wilkins Centre for Biodiscovery (M.P.H.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Mechanisms of resistance (and sensitivity) of hypoxic cells to cytotoxic therapy (PDF 428 kb)
Glossary
- Bioreductive prodrugs
-
Biologically inactive molecules that are converted to an active drug by enzymatic reduction.
- Superoxide
-
A free radical formed by a one-electron reduction of oxygen, including by electron transfer from a prodrug free radical. Despite its name, superoxide itself is not highly reactive and is generally less toxic than the reduced prodrug, so its generation represents a detoxification mechanism in aerobic cells.
- Replication fork
-
The branch-point structure that forms between two DNA template strands during DNA replication at which nascent DNA synthesis is ongoing.
- Homologous recombination
-
(HR). High-fidelity repair of DNA lesions, including double-strand breaks, in S and G2 phases of the cell cycle, using a sister chromatid as a template.
- Multicellular spheroids
-
Spherical clusters of cells that grow large enough to become diffusion-limited, and thus model some features of the tumour microenvironment.
- Multicellular layers
-
(MCLs). Three-dimensional cell cultures that model the extravascular compartment of tumours. Grown on collagen-coated micro-porous membranes, they allow measurement of drug diffusion and metabolism in tumour-like tissue.
- Bystander effect
-
In the context of bioreductive prodrugs, the killing of adjacent cells that lack prodrug-activating ability through local diffusion of the active drug.
- Pseudo-hypoxia
-
The induction of molecular responses analogous to those caused by hypoxia but triggered by other conditions.
- Cap-dependent translation
-
Translation initiated by binding of the eIF4F complex to the methyl-7-G(5′)pppN structure (cap) at the 5′ end of the mRNA.
- Synthetic lethal interaction
-
In genetics, an interaction between two non-lethal mutations that, in combination, confer lethality. In chemical genetics, this term can refer to interaction between a drug and mutation that confers greater drug-sensitivity than with the wild type.
- Autochthonous tumours
-
Tumours that arise in the host being studied, as distinct from tumours introduced by transplantation.
- Network medicine
-
Analysis of biological networks to derive understanding of disease and therapy.
Rights and permissions
About this article
Cite this article
Wilson, W., Hay, M. Targeting hypoxia in cancer therapy. Nat Rev Cancer 11, 393–410 (2011). https://doi.org/10.1038/nrc3064
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3064
This article is cited by
-
The progressive trend of modeling and drug screening systems of breast cancer bone metastasis
Journal of Biological Engineering (2024)
-
MicroRNA-561-3p indirectly regulates the PD-L1 expression by targeting ZEB1, HIF1A, and MYC genes in breast cancer
Scientific Reports (2024)
-
HIF-2α-dependent TGFBI promotes ovarian cancer chemoresistance by activating PI3K/Akt pathway to inhibit apoptosis and facilitate DNA repair process
Scientific Reports (2024)
-
Hypoxia-activated XBP1s recruits HDAC2-EZH2 to engage epigenetic suppression of ΔNp63α expression and promote breast cancer metastasis independent of HIF1α
Cell Death & Differentiation (2024)
-
Exploring chronic and transient tumor hypoxia for predicting the efficacy of hypoxia-activated pro-drugs
npj Systems Biology and Applications (2024)