Abstract
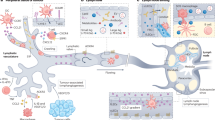
Malignant tumours can spread to lymph nodes through lymphatic vessels. Recent studies show that tumours produce a range of growth factors that directly or indirectly stimulate lymphatic vessel growth (lymphangiogenesis) and lymphatic metastasis. These findings indicate that tumour lymphangiogenesis, similar to haemangiogenesis, is a complex process that is regulated by multiple growth factors. Understanding the underlying mechanisms by which tumours induce lymphangiogenesis might provide important information for the therapeutic intervention of metastatic spread.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Etzioni, R. et al. The case for early detection. Nature Rev. Cancer 3, 243–252 (2003).
Kuroda, H., Sakamoto, G., Ohnisi, K. & Itoyama, S. Clinical and pathologic features of invasive micropapillary carcinoma. Breast Cancer 11, 169–174 (2004).
Moskowitz, M. et al. Breast cancer screening. Preliminary report of 207 biopsies performed in 4,128 volunteer screenees. Cancer 36, 2245–2250 (1975).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Porter, G. J. et al. Patterns of metastatic breast carcinoma: influence of tumour histological grade. Clin. Radiol. 59, 1094–1098 (2004).
van't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Fujisawa, T., Yamaguchi, Y., Saitoh, Y., Hiroshima, K. & Ohwada, H. Blood and lymphatic vessel invasion as prognostic factors for patients with primary resected nonsmall cell carcinoma of the lung with intrapulmonary metastases. Cancer 76, 2464–2470 (1995).
Taubert, H. et al. Detection of disseminated tumor cells in peripheral blood of patients with breast cancer: correlation to nodal status and occurrence of metastases: lymphogenous and hematogenous metastasis of Lewis lung carcinoma in the mouse. Gynecol. Oncol. 92, 256–261 (2004).
Weiss, L. & Ward, P. M. Lymphogenous and hematogenous metastasis of Lewis lung carcinoma in the mouse. Int. J. Cancer 40, 570–574 (1987).
Achen, M. G., McColl, B. K. & Stacker, S. A. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell 7, 121–127 (2005).
Stacker, S. A., Achen, M. G., Jussila, L., Baldwin, M. E. & Alitalo, K. Lymphangiogenesis and cancer metastasis. Nature Rev. Cancer 2, 573–583 (2002).
Pepper, M. S. & Skobe, M. Lymphatic endothelium: morphological, molecular and functional properties. J. Cell Biol. 163, 209–213 (2003).
Witte, M. H. & Witte, C. L. Lymphatics and blood vessels, lymphangiogenesis and hemangiogenesis: from cell biology to clinical medicine. Lymphology 20, 257–266 (1987).
Leak, L. V. Studies on the permeability of lymphatic capillaries. J. Cell Biol. 50, 300–323 (1971).
Saharinen, P., Tammela, T., Karkkainen, M. J. & Alitalo, K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 25, 387–395 (2004).
Isaka, N., Padera, T. P., Hagendoorn, J., Fukumura, D. & Jain, R. K. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 64, 4400–4404 (2004).
Padera, T. P. et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296, 1883–1886 (2002).
Kim, U., Park, H. C. & Choi, K. H. Differential permeability of lymphatic and blood vessels in determining the route of metastasis as demonstrated by indirect lymphography. Clin. Exp. Metastasis 6, 291–299 (1988).
Alitalo, K., Mohla, S. & Ruoslahti, E. Lymphangiogenesis and cancer: meeting report. Cancer Res 64, 9225–9229 (2004).
Skobe, M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Med. 7, 192–198 (2001).
Cao, R. et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6, 333–345 (2004).
Chang, L., Kaipainen, A. & Folkman, J. Lymphangiogenesis new mechanisms. Ann. NY Acad. Sci. 979, 111–119 (2002).
Chang, L. K. et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc. Natl Acad. Sci. USA 101, 11658–11663 (2004).
Gale, N. W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev. Cell 3, 411–423 (2002).
Kubo, H. et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl Acad. Sci. USA 99, 8868–8873 (2002).
Veikkola, T. & Alitalo, K. Dual role of Ang2 in postnatal angiogenesis and lymphangiogenesis. Dev. Cell 3, 302–304 (2002).
Vincent, L. & Rafii, S. Vascular frontiers without borders: multifaceted roles of platelet-derived growth factor (PDGF) in supporting postnatal angiogenesis and lymphangiogenesis. Cancer Cell 6, 307–309 (2004).
Maeshima, Y. et al. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science 295, 140–143 (2002).
O'Reilly, M. S. et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 (1994).
O'Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1, 27–31 (1995).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Stacker, S. A. et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature Med. 7, 186–191 (2001).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl J. Med. 285, 1182–1186 (1971).
Folkman, J. Seminars in medicine of the beth israel hospital, boston. Clinical applications of research on angiogenesis. N. Engl J. Med. 333, 1757–1763 (1995).
Relf, M. et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 57, 963–969 (1997).
Kandel, J. et al. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell 66, 1095–1104 (1991).
Galy, B., Creancier, L., Prado-Lourenco, L., Prats, A. C. & Prats, H. p53 directs conformational change and translation initiation blockade of human fibroblast growth factor 2 mRNA. Oncogene 20, 4613–4620 (2001).
Galy, B., Creancier, L., Zanibellato, C., Prats, A. C. & Prats, H. Tumour suppressor p53 inhibits human fibroblast growth factor 2 expression by a post-transcriptional mechanism. Oncogene 20, 1669–1677 (2001).
Ueba, T. et al. Transcriptional regulation of basic fibroblast growth factor gene by p53 in human glioblastoma and hepatocellular carcinoma cells. Proc. Natl Acad. Sci. USA 91, 9009–9013. (1994).
Enholm, B. et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 14, 2475–2483 (1997).
Gjerset, R. A. et al. Characterization of a new human glioblastoma cell line that expresses mutant p53 and lacks activation of the PDGF pathway. In Vitro Cell. Dev. Biol. Anim. 31, 207–214 (1995).
Uramoto, H. et al. pRb, Myc and p53 are critically involved in SV40 large T antigen repression of PDGF β-receptor transcription. J. Cell Sci. 117, 3855–3865 (2004).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Makino, Y. et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414, 550–554 (2001).
Sano, T. & Horiguchi, H. Von Hippel–Lindau disease. Microsc. Res. Tech. 60, 159–164 (2003).
Arbiser, J. L. et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc. Natl Acad. Sci. USA 94, 861–866 (1997).
Su, W. C. et al. Expression of oncogene products HER2/Neu and Ras and fibrosis-related growth factors bFGF, TGF-β, and PDGF in bile from biliary malignancies and inflammatory disorders. Dig. Dis. Sci. 46, 1387–1392 (2001).
Lohela, M., Saaristo, A., Veikkola, T. & Alitalo, K. Lymphangiogenic growth factors, receptors and therapies. Thromb. Haemost. 90, 167–184 (2003).
Petrova, T. V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Heldin, C. H., Rubin, K., Pietras, K. & Ostman, A. High interstitial fluid pressure — an obstacle in cancer therapy. Nature Rev. Cancer 4, 806–813 (2004).
Thurston, G. et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286, 2511–2514 (1999).
Cliff, W. J. & Nicoll, P. A. Structure and function of lymphatic vessels of the bat's wing. Q. J. Exp. Physiol. Cogn. Med. Sci. 55, 112–131 (1970).
Nagy, J. A. et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J. Exp. Med. 196, 1497–1506 (2002).
Beasley, N. J. et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 62, 1315–1320 (2002).
Cao, Y. Direct role of PDGF-BB in lymphangiogenesis and lymphatic metastasis. Cell Cycle 4, 228–230 (2005).
Dadras, S. S. et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am. J. Pathol. 162, 1951–1960 (2003).
Achen, M. G. et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl Acad. Sci. USA 95, 548–553 (1998).
Mandriota, S. J. et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20, 672–682 (2001).
Choi, W. W. et al. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod. Pathol. 18, 143–152 (2005).
Cursiefen, C. et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol. Vis. Sci. 45, 2666–2673 (2004).
Joukov, V. et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 290–298 (1996).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Salven, P., Mustjoki, S., Alitalo, R., Alitalo, K. & Rafii, S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood 101, 168–172 (2003).
He, Y. et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 64, 3737–3740 (2004).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature Immunol. 5, 74–80 (2004).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Dvorak, H. F. Rous-whipple award lecture. How tumors make bad blood vessels and stroma. Am. J. Pathol. 162, 1747–1757 (2003).
Lyden, D. et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 7, 1194–1201 (2001).
Cao, R. et al. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 94, 664–670 (2004).
Baluk, P. et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 115, 247–257 (2005).
Cursiefen, C. et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113, 1040–1050 (2004).
Sawano, A. et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97, 785–791 (2001).
Fredriksson, L., Li, H. & Eriksson, U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 15, 197–204 (2004).
Westermark, B. & Heldin, C. H. Platelet-derived growth factor. Structure, function and implications in normal and malignant cell growth. Acta. Oncol. 32, 101–105 (1993).
Betsholtz, C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 15, 215–228 (2004).
Hoch, R. V. & Soriano, P. Roles of PDGF in animal development. Development 130, 4769–4784 (2003).
Lindahl, P., Johansson, B. R., Leveen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242–245 (1997).
Anan, K. et al. Vascular endothelial growth factor and platelet-derived growth factor are potential angiogenic and metastatic factors in human breast cancer. Surgery 119, 333–339 (1996).
Travers, M. T. et al. Growth factor expression in normal, benign, and malignant breast tissue. Br. Med. J. (Clin. Res. Ed.) 296, 1621–1624 (1988).
Lamszus, K., Heese, O. & Westphal, M. Angiogenesis-related growth factors in brain tumors. Cancer Treat. Res. 117, 169–190 (2004).
Ostman, A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 15, 275–286 (2004).
Roberts, W. G. et al. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine kinase inhibitor, CP-673,451. Cancer Res. 65, 957–966 (2005).
Shing, Y. et al. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science 223, 1296–1299 (1984).
Javerzat, S., Auguste, P. & Bikfalvi, A. The role of fibroblast growth factors in vascular development. Trends Mol. Med. 8, 483–489 (2002).
Cao, Y. H. & Pettersson, R. F. Human acidic fibroblast growth factor overexpressed in insect cells is not secreted into the medium. Growth Factors 3, 1–13 (1990).
Friesel, R. E. & Maciag, T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J. 9, 919–925 (1995).
Malecki, J., Wesche, J., Skjerpen, C. S., Wiedlocha, A. & Olsnes, S. Translocation of FGF-1 and FGF-2 across vesicular membranes occurs during G1-phase by a common mechanism. Mol. Biol. Cell 15, 801–814 (2004).
Christofori, G. & Luef, S. Novel forms of acidic fibroblast growth factor-1 are constitutively exported by b tumor cell lines independent from conventional secretion and apoptosis. Angiogenesis 1, 55–70 (1997).
Nguyen, M. et al. Elevated levels of an angiogenic peptide, basic fibroblast growth factor, in the urine of patients with a wide spectrum of cancers. J. Natl Cancer Inst. 86, 356–361 (1994).
Tille, J. C., Nisato, R. & Pepper, M. S. Lymphangiogenesis and tumour metastasis. Novartis Found. Symp. 256, 112–131 (2004).
Rutanen, J. et al. Vascular endothelial growth factor-D expression in human atherosclerotic lesions. Cardiovasc. Res. 59, 971–979 (2003).
Schoppmann, S. F. et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 161, 947–956 (2002).
Yancopoulos, G. D. et al. Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 (2000).
Karkkainen, M. J. et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl Acad. Sci. USA 98, 12677–12682 (2001).
Morisada, T. et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 105, 4649–4656 (2005).
Mouta, C. & Heroult, M. Inflammatory triggers of lymphangiogenesis. Lymphat. Res. Biol. 1, 201–218 (2003).
Atkins, C. D. Re: Influence of the new AJCC breast cancer staging system on sentinel lymph node positivity and false-negative rates. J. Natl Cancer Inst. 96, 1639 (2004).
Delahaye, S. et al. [Routine sentinel node detection in breast cancer. Experience of the Curie Institute]. Bull Cancer 91, 641–647 (2004) (in French).
Nakamura, E. S., Koizumi, K., Kobayashi, M. & Saiki, I. Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci. 95, 25–31 (2004).
Cao, R. et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nature Med. 9, 604–613 (2003).
Acknowledgements
Research grants of Y.C.'s laboratory are received from the Swedish Research Council, the Swedish Heart and Lung Foundation, the Swedish Cancer Foundation, the Karolinska Institute fund, the Söderberg Foundation, the EU integrated projects of Angiotargeting, and VascuPlug. Y.C. is supported by the Swedish Research Council.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Cao, Y. Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer 5, 735–743 (2005). https://doi.org/10.1038/nrc1693
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc1693
This article is cited by
-
Loss of TTC17 promotes breast cancer metastasis through RAP1/CDC42 signaling and sensitizes it to rapamycin and paclitaxel
Cell & Bioscience (2023)
-
Exosomal circ_0026611 contributes to lymphangiogenesis by reducing PROX1 acetylation and ubiquitination in human lymphatic endothelial cells (HLECs)
Cellular & Molecular Biology Letters (2023)
-
Aiphanol, a multi-targeting stilbenolignan, potently suppresses mouse lymphangiogenesis and lymphatic metastasis
Acta Pharmacologica Sinica (2023)
-
Efficacy of concurrent chemoradiotherapy plus Endostar compared with concurrent chemoradiotherapy in the treatment of locally advanced nasopharyngeal carcinoma: a retrospective study
Radiation Oncology (2022)
-
GPR182 limits antitumor immunity via chemokine scavenging in mouse melanoma models
Nature Communications (2022)