Abstract
All FDA-approved antipsychotic drugs (APDs) target primarily dopamine D2 or serotonin (5-HT2A) receptors, or both; however, these medications are not universally effective, they may produce undesirable side effects, and provide only partial amelioration of negative and cognitive symptoms. The heterogeneity of pharmacological responses in schizophrenic patients suggests that additional drug targets may be effective in improving aspects of this syndrome. Recent evidence suggests that 5-HT2C receptors may be a promising target for schizophrenia since their activation reduces mesolimbic nigrostriatal dopamine release (which conveys antipsychotic action), they are expressed almost exclusively in CNS, and have weight-loss-promoting capabilities. A difficulty in developing 5-HT2C agonists is that most ligands also possess 5-HT2B and/or 5-HT2A activities. We have developed selective 5-HT2C ligands and herein describe their preclinical effectiveness for treating schizophrenia-like behaviors. JJ-3-45, JJ-3-42, and JJ-5-34 reduced amphetamine-stimulated hyperlocomotion, restored amphetamine-disrupted prepulse inhibition, improved social behavior, and novel object recognition memory in NMDA receptor hypofunctioning NR1-knockdown mice, and were essentially devoid of catalepsy. However, they decreased motivation in a breakpoint assay and did not promote reversal learning in MK-801-treated mice. Somewhat similar effects were observed with lorcaserin, a 5-HT2C agonist with potent 5-HT2B and 5-HT2A agonist activities, which is approved for treating obesity. Microdialysis studies revealed that both JJ-3-42 and lorcaserin reduced dopamine efflux in the infralimbic cortex, while only JJ-3-42 decreased it in striatum. Collectively, these results provide additional evidence that 5-HT2C receptors are suitable drug targets with fewer side effects, greater therapeutic selectivity, and enhanced efficacy for treating schizophrenia and related disorders than current APDs.
Similar content being viewed by others
Introduction
Atypical antipsychotic drugs (APDs) are the first choice for treating schizophrenia (SZ) and they act primarily through blockade or partial antagonism of dopamine (DA) D2 receptors and inverse agonism at serotonin 2A (5-HT2A) receptors, together with activities at other DA and 5-HT receptors (Meltzer et al, 1989; Roth et al, 2004). Psychotic disorders include SZ, schizoaffective disorder, bipolar disorder, major depression, and various neurological conditions (Arciniegas et al, 2001; Wigman et al, 2012; Landqvist-Waldö et al, 2015). The limited efficacy of atypical APDs for treating negative symptoms and cognitive impairment in SZ, and the role of N-methyl-D-aspartate receptor (NMDAR) antagonists to induce cognitive deficiency in man and laboratory animals implicates glutamate (Glu) and γ-aminobutyric acid (GABA) receptors in some aspects of SZ (López-Gil et al, 2010). It is now established that atypical APDs are superior to selective D2 antagonists for improving cognitive function in SZ (Désaméricq et al, 2014; Meltzer, 2015), while having modest effects on negative symptoms (Leucht et al, 1999; Miyamoto et al, 2005).
There is some evidence that 5-HT2C agonists have antipsychotic efficacy (Marquis et al, 2007). This receptor interacts with Gq/11 protein to activate phospholipase Cβ, leading to intracellular calcium release (Conn et al, 1986; McCorvy and Roth, 2015). In rodents, 5-HT2C mRNA is present in dorsal and ventral striatum and is coexpressed primarily in substantia nigra and ventral tegmental area (VTA) neurons containing glutamic acid decarboxylase mRNA (Bubar et al, 2011; Eberle-Wang et al, 1997), where the 5-HT2C receptor may indirectly inhibit DA neurons through GABA interneuronal activation. Neurochemical and electrophysiological studies confirm this point, as a 5-HT2C inverse agonist increases firing in VTA and substantia nigra, enhances VTA bursting activity, and leads to DA release in the nucleus accumbens (NAc) and striatum—with more pronounced effects on DA mesolimbic neurons (Gobert et al, 2000). Intrastriatal administration of this inverse agonist increases DA release in the lateral striatum (Alex et al, 2005). Conversely, the 5-HT2C agonist Ro-60-0175 decreases DA release in the NAc and striatum (Gobert et al, 2000). This agonist blocks cocaine-induced open field hyperactivity and the selective 5-HT2C antagonist SB 242 084 reverses this effect (Grottick et al, 2000). However, Ro-60-0175 fails to alter cocaine-induced DA release and a 5-HT2C inverse agonist does not influence amphetamine (AMPH)-stimulated DA release in NAc or striatum of halothane-anesthetized rats (Porras et al, 2002; Navailles et al, 2004). Hence, the effects of 5-HT2C agonists may involve striatal non-dopaminergic postsynaptic mechanisms. Further evidence for a modulatory role of 5-HT2C receptors on DA function derives from Htr2c disruption, resulting in elevated DA release in dorsal striatum and enhanced AMPH-induced stereotypies in mice (Abdallah et al, 2009). In humans, the HTR2c polymorphism Cys23Ser is associated with augmented striatal DA release during pain stress (Mickey et al, 2012). Since non-medicated SZ patients may have elevated DA synthesis and release in caudate (Kegeles et al, 2010), 5-HT2C ligands may represent a new generation of APDs that modulate dopaminergic neurotransmission in the striatum.
Recently, we have published the synthesis and initial behavioral characterization of novel 5-HT2C agonists with promising APD properties in mouse models of SZ (Cheng et al, 2015, 2016). SZ is a neurodevelopmental disorder characterized by positive and negative symptoms, and cognitive impairment. There is an urgent need to control APD-induced side effects, such as neuroleptic malignant syndrome, psychomotor retardation, weight gain, and blood and bone marrow complications. Notably, novel 5-HT2C ligands show promise in treating obesity (Smith et al, 2010) and improving glycemic control in type 2 diabetes patients (O'Neil et al, 2012). Preclinical studies suggest possible procognitive actions of 5-HT2C agonists in novel object recognition memory (NORM) (Cheng et al, 2016).
The present study examined effects of three novel compounds with selective 5-HT2C agonism in pharmacological and genetic models of SZ. For positive symptoms, AMPH-induced hyperactivity and disruption of prepulse inhibition (PPI) were used. For negative symptoms, we focused on motivational impairment and avolition using a breakpoint assay. We also used the sociability test with the NMDAR hypofunctioning NR1-knockdown (NR1-KD) mice (Mohn et al, 1999; Milenkovic et al, 2014; Park et al, 2016). For cognition we included NORM and a visual discrimination reversal test. For possible extrapyramidal side effects, we used the horizontal bar test for catalepsy. Finally, we measured the effects of one of the compounds on extracellular levels of neurotransmitters in the infralimbic cortex and striatum of mice.
Materials and methods
Detailed descriptions of the methods and procedures can be found in the Supplementary Information.
Animals
Adult male and female C57BL/6J and female C3H/HeJ mice were purchased (Jackson Laboratories, Bar Harbor, ME), whereas wild-type (WT) and NR1-KD mice were bred and maintained as described (Park et al, 2016). Mice were housed 3–5 per cage on a 14 : 10 h light : dark cycle (Duke: lights on 0700 h) for behavioral studies and on a 12 : 12 h light : dark cycle (Northwestern: lights on 0500 h) for microdialysis experiments, with food and water provided ad libitum. Six cohorts were used: cohort 1, open field activity and PPI; cohort 2, motivation; cohort 3, sociability and NORM; cohort 4, visual discrimination learning and reversal; cohort 5, catalepsy; and cohort 6, microdialysis. At least 10 days were interposed between tests within any cohort. All experiments were conducted with approved protocols from the Duke and Northwestern Universities Institutional Animal Care and Use Committees.
Drugs
The lorcaserin (Selleckchem LLC, Houston, TX), AMPH (Sigma-Aldrich, St Louis MO), MK-801 (Sigma-Aldrich), and JJ-3-45, JJ-3-42, and JJ-5-34 were reconstituted in sterile 0.9% saline. Clozapine (Sigma-Aldrich) was dissolved in 0.2% glacial acetic acid with saline, whereas haloperidol (Sigma-Aldrich) was dissolved to a final concentration of 3% dimethylsulfoxide. All treatments were administered (intraperitoneally) in 5 ml/kg volume, except as noted.
Open Field Activity
C57BL/6J mice were injected with vehicle or different doses of the 5-HT2C-selective compounds or lorcaserin, placed into the open field (Omnitech, Columbus, OH) for 15 min, removed and injected with vehicle or 3 mg/kg AMPH, and immediately returned to the apparatus for 105 min. Activity was monitored as cumulative distance traveled.
Prepulse Inhibition
C57BL/6J mice were administered vehicle, different doses of the 5-HT2C-selective compounds, or lorcaserin in their home cage. Ten minutes later, animals were given vehicle or 3 mg/kg AMPH and acclimated to a 65 dB white-noise background in the PPI apparatus (San Diego Instruments, San Diego, CA). Five minutes later, mice were presented with combinations of startle (120 dB), prepulse-pulse (4, 8, and 12 dB over the 65 dB background followed by 120 dB), and null trials over 25 min (Park et al, 2016). Activity was recorded during all trials. The data were presented as percent PPI=(1−(prepulse trials/startle-only trials)) × *100, and as startle and null activities.
Breakpoint as an Index of Motivation
Throughout the experiment mice were food restricted to 85–90% of baseline body weight and housed 4/cage. Mice were trained initially on a continuous reinforcement schedule with condensed milk as a reward. Subsequently, animals were trained on a progressive ratio schedule where the number of bar presses required to earn the reward doubled. Once performance was stable, mice were injected with vehicle, the JJ compounds, or lorcaserin and tested. Breakpoint (highest ratio completed) was recorded.
Sociability
There were three phases to this test: identical non-social (control), a social and non-social (social affiliation), and a novel and familiar social pairing (social preference). Female C3H/HeJ mice were used as partners with WT and NR1-KD tester mice. The tester NR1 mice were injected with vehicle, 0.5 mg/kg clozapine (positive control), or different doses of the 5-HT2C-selective compounds or lorcaserin. Ten minutes later, individual tester NR1 mice were evaluated sequentially in 10 min tests for responses to two identical non-social stimuli (empty wire-mesh cages), to novel social (C3H/HeJ mouse) and non-social stimuli, and to novel social (C3H/HeJ mouse) and now familiar social (previous C3H/HeJ mouse) stimuli. The duration of stimulus contacts was monitored. Preference scores ((time spent with one stimulus−time spent with the other stimulus)/total time spent exploring both stimuli) were calculated where positive scores indicated a preference for the non-social stimulus in the first test, for the social stimulus in the second test, and for the novel social stimulus in the third test.
Novel Object Recognition Memory
Before training, WT and NR1-KD mice were administered the vehicle or different doses of the 5-HT2C-selective compounds or lorcaserin, and 30 min later, they were presented with identical object pairs for 5 min. The next day mice were administered the same treatments and were tested after 30 min. Here, one of the now familiar objects was replaced with a novel object and the mice were tested over 5 min. The duration of object contracts was calculated as a control. Recognition scores ((time spent with novel object−time spent with familiar object)/total time with both objects) were calculated where positive scores signified novel object preference, negative scores indicated familiar object preference, and scores approaching ‘zero’ denoted no object preference.
Reversal of Visual Discrimination Learning
Since behavioral responses across tests were similar for the three JJ compounds, we only evaluated JJ-3-45 and selected the 2 mg/kg dose because this was the highest dose that did not disrupt the visual discrimination and was close to the optimal dose for NORM. Touchscreens were used to display two separate images for visual discrimination and reversal (Med Associates).
Throughout the experiment mice were food-restricted to 85–90% of baseline body weight and housed 3–5 mice per cage. At training mice were taught to nose poke at white rectangles to receive a condensed milk (Kroger) reward. Subsequently, mice were trained on a simultaneous discrimination where one of two stimuli was reinforced. Upon reaching a criterion of 80% correct responses, the mouse was given MK-801 or JJ-3-45 to determine which doses would not perturb its visual discrimination performance. Subsequently, mice were assigned to vehicle, MK-801, and MK-801 plus JJ-3-45 groups and these treatments were chronically administered. The mice were tested in the visual discrimination for two additional sessions and then the reward contingencies were reversed. Responses were scored as the number of correct trials, number of errors, and percent correct ((correct/total trials) × 100).
Catalepsy
Baseline responses were recorded and then separate groups of C57BL/6J mice were injected with haloperidol, different doses of the three JJ compounds, or lorcaserin. Mice were tested 60 min later for catalepsy. The latency for a mouse to remove its paws from the bar was recorded as an index of catalepsy with a maximum time of 90 s.
Microdialysis Procedure and Analyses of Neurotransmitters
C57BL/6J males were housed in groups of 3–5 per cage until surgery; afterwards, they were housed individually. Mice were anesthetized with 1.5% isoflurane (Isothesia; Butler-Schein, Dublin, OH) and cannulated with dummy probes in the infralimbic cortex and ventral striatum. Two days later, microdialysis probes were inserted on the morning of the experiment with perfusion at 1 μl/min. Two hours later, microdialysis samples were collected every 30 min. After 90 min, mice were injected with vehicle, 10 mg/kg JJ-3-42, or 1 mg/kg lorcaserin and samples were collected over another 180 min. Parenthetically, the doses for JJ-3–42 and lorcaserin were based on the open field experiment. Samples were submitted to mass spectrometry for acetylcholine (ACh), DA, norepinephrine, 3,4-dihydroxyphenylacetic acid (DOPAC); homovanillic acid (HVA), 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), Glu, and GABA. Baseline was designated as 100% and subsequent neurochemical levels were expressed as percent baseline.
Statistical Analyses
The data were presented as means and standard errors of the mean. One-, two-way, or repeated-measures ANOVA (RMANOVA) were used with Dunnett or Bonferroni post hoc analyses. Owing to variability in the neurochemistry, some data were analyzed by Kruskal–Wallis, followed by Mann–Whitney tests. A P<0.05 was considered significant.
Results
AMPH-Stimulated Hyperlocomotion in C57BL/6J Mice
AMPH-stimulated hyperlocomotion is used as a model of hyperdopaminergia in rodents and APDs reduce it (Geyer and Moghaddam, 2002). JJ-3-45, JJ-3-42, JJ-5-34, or lorcaserin were injected and baseline activity was assessed over the first 15 min. Relative to vehicle, basal locomotion was attenuated by 30 mg/kg JJ-3-45 (P<0.001; Figure 1a) and 5 mg/kg JJ-3-42 (P<0.042; Figure 1b), but it was increased with 0.25 mg/kg lorcaserin compared with all other groups (P-values<0.014; Figure 1d).
All three JJ compounds and lorcaserin reduced the 3 mg/kg amphetamine-stimulated hyperlocomotion in the open field and restored amphetamine-disrupted prepulse inhibition (PPI) in C57BL/6 mice. (a–d) Open field baseline locomotor activity (0–15 min) in mice administered JJ-3-45, JJ-3-42, JJ-5-34, or lorcaserin. A one-way analysis of variance (ANOVA) observed a main effect of dose: JJ-3-45 (F(5,81)=6.740, P<0.001), JJ-3-42 (F(4,75)=2.604, P<0.042), JJ-5-34 (F(4,62)=3.511, P<0.012), and lorcaserin (F(4,46)=6.892, P<0.001). (e–h) Amphetamine-stimulated hyperlocomotion (105 min) with dose responses for JJ-3-45, JJ-3-42, JJ-5-34, and lorcaserin. A one-way ANOVA noted a main effect of dose: JJ-3-45 (F(5,81)=14.718, P<0.001), JJ-3-42 (F(4,75)=18.232, P<0.001), JJ-5-34 (F(4,62)=15.448, P<0.001), and lorcaserin (F(4,46)=9.987, P<0.001). (i–l) Dose responses for JJ-3-45, JJ-3-42, JJ-5-34, and lorcaserin in PPI. A repeated-measures ANOVA (RMANOVA) within-subjects effects for PPI intensity (F(2,242)=315.229, P<0.001); between subjects effects for treatment (F(14,121)=5.302, P<0.001). Groups: V–V, vehicle–vehicle control; V–A, vehicle–amphetamine. N=9–31 mice per compound or drug dose in the open field study and N=8–15 mice per compound or drug dose in the PPI experiment; *P<0.001, compared with the V–V control; ^P<0.01, compared with the V–A group.
After baseline assessment, vehicle or AMPH (3 mg/kg, intaperitoneally) was injected and mice were returned to the open field for 105 min. The 20 and 30 mg/kg JJ-3-45 decreased the AMPH-induced hyperactivity (P-values<0.045), such that responses to the highest dose were similar to the vehicle control (Figure 1e). Similarly, 10 and 20 mg/kg JJ-3-42 suppressed hyperlocomotion (P-values<0.050) to levels statistically indistinguishable from vehicle controls (Figure 1f). By comparison, only 20 mg/kg JJ-5-34 reduced AMPH-induced hyperlocomotion (P<0.001) to the vehicle control (Figure 1g). Thus, all novel 5-HT2C compounds antagonized the AMPH-stimulated response. By contrast, lorcaserin failed to inhibit the AMPH-stimulated effect until the highest dose was used (P<0.001; Figure 1h).
AMPH-Disrupted PPI in C57BL/6J Mice
PPI is reduced in SZ and APDs can normalize AMPH-disrupted PPI in rodents (Krystal et al, 1999; Geyer and Moghaddam, 2002). JJ-3-45, JJ-3-42, JJ-5-34, or lorcaserin were administered, 10 min later the vehicle or AMPH (3 mg/kg, i.p.) were given, and the mice were tested after 5 min. AMPH depressed PPI compared with the vehicle control (P<0.001; Figure 1i–l). All compounds reversed this disruptive effect as demonstrated with 0.5 mg/kg JJ-3-45; 1, 2, and 4 mg/kg JJ-3-42 and JJ-5-34; and 0.5 and 1 mg/kg lorcaserin (P-values<0.006). Startle activity varied with treatment and this was due to a dose-induced reduction in responses (Table 1); however, the post hoc tests were not significant. Null (baseline) activity was significantly enhanced with 0.1 and 1 mg/kg lorcaserin (P-values<0.030; Table 1). Thus, all selective 5-HT2C compounds and lorcaserin restored the AMPH-disrupted PPI.
Breakpoint as an Index of Motivation in C57BL/6 Mice
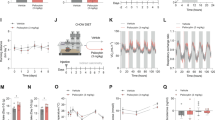
Motivation is regulated by mesolimbic dopaminergic neurons and is reduced in SZ (Salamone and Correa, 2012). Mice were tested on a progressive ratio schedule for food reinforcement as an index of motivation. They were treated with the vehicle and different doses of the JJ compounds or lorcaserin. The 1 and 5 mg/kg doses of JJ-3-45 significantly decreased the breakpoint relative to the vehicle control (P-values<0.001) (Figure 2a). Similarly, all doses of JJ-3-42 and JJ-5-34 decreased the breakpoints (P-values<0.009) (Figures 2b and c). Lorcaserin depressed lever responding beginning at 0.5 mg/kg (P<0.029) with higher doses exerting greater effects on breakpoint (P-values<0.001) (Figure 2d). Hence, all JJ compounds and lorcaserin reduced breakpoints in dose-dependent manner.
The JJ compounds and lorcaserin decreased lever pressing under a progressive ratio schedule for food reinforcement in C57BL/6 mice, but increased social behavior in NR1-knockdown (NR1-KD) mice. (a–d) Dose–responses for JJ-3-45, JJ-3-42, JJ-5-34, and lorcaserin are shown as breakpoints for assessment of motivation. A repeated-measures analysis of variance (RMANOVA) for JJ-3-45 within-subjects effect of session (F(1,39)=31.395, P<0.001); session by dose interaction (F(3,39)=4.898, P<0.01). A RMANOVA for JJ-3-42 within-subjects effect of session (F(1,42)=121.664, P<0.001); session by dose interaction (F(3,42)=4.103, P<0.012). A RMANOVA for JJ-5-34 within-subjects effect of session (F(1,42)=50.513, P<0.001). A RMANOVA for lorcaserin within subjects effect for session (F(1,40)=57.079, P<0.001), session by dose interaction (F(3,40)=8.876, P<0.001); between-subjects effect of treatment (F(3,40)=2.746, P=0.055). (e and f) In the sociability test, mice were subjected to three test phases involving pairings of two identical non-social stimuli, a novel social and a non-social stimulus, and familiar and novel social stimuli. (e) Experiments were conducted with wild-type (WT) mice administered the vehicle, clozapine (CLZ), or different doses of JJ-3-45, JJ-3-42, JJ-5-34, and lorcaserin. (f) The same treatment conditions were imposed on NR1-KD mice. A RMANOVA for the vehicle condition: within-subjects effect of test phase (F(2,30)=17.896, P<0.001), test phase by genotype interaction (F(2,30)=10.584, P<0.001); between-subjects effect of genotype (F(1,15)=54.524, P<0.001). A RMANOVA for the vehicle vs CLZ: within-subjects effect of test phase (F(2,58)=37.586, P<0.001), test phase by genotype (F(2,58)=3.595, P=0.034), and test phase by genotype by treatment (F(2,58)=9.284, P<0.001) interactions; between-subjects effect of genotype (F(1,29)=20.688, P<0.001), genotype by treatment interaction (F(1,29)=46.142, P<0.001). A RMANOVA for the vehicle and CLZ vs JJ-3–45: within-subjects effect of test phase (F(2,118)=71.591, P<0.001), test phase by treatment (F(6,118)=2.157, P=0.052) and test phase by genotype by treatment (F(6,118)=3.373, P=0.004) interactions; between-subjects effects of genotype (F(1,59)=12.307, P<0.001) and treatment (F(3,59)=2.780, P=0.049), genotype by treatment interaction (F(3,59)=13.728, P<0.001). A RMANOVA for the vehicle and CLZ vs JJ-3-42: within-subjects effect of test phase (F(2,118)=90.784, P<0.001), and the test phase by genotype (F(2,118)=3.512, P=0.033), test phase by treatment (F(6,118)=3.737, P=0.002), and test phase by genotype by treatment (F(6,118)=3.209, P=0.006) interactions; between-subjects effects of genotype (F(1,59)=10.208, P=0.002) and treatment (F(3,59)=3.336, P=0.025), genotype by treatment interaction (F(3,59)=15.487, P<0.001). A RMANOVA for the vehicle and CLZ vs JJ-5-34: within-subjects effect of test phase (F(2,118)=82.860, P<0.001), and the test phase by genotype (F(2,118)=3.964, P=0.022), test phase by treatment (F(6,118)=2.632, P=0.020), and test phase by genotype by treatment (F(6,118)=5.779, P<0.001) interactions; between-subjects effects of genotype (F(1,59)=22.227, P<0.001) and treatment (F(3,59)=3.255, P=0.028), genotype by treatment interaction (F(3,59)=19.790, P<0.001). A RMANOVA for the vehicle and CLZ vs lorcaserin: within-subjects effect of test phase (F(2,114)=85.422, P<0.001), test phase by treatment (F(6,114)=3.208, P=0.006) and test phase by genotype by treatment (F(6,114)=7.020, P<0.001) interactions; between-subjects effect of genotype (F(1,57)=6.775, P=0.012), genotype by treatment interaction (F(3,57)=28.218, P<0.001). N=8–13 mice per compound or drug dose in the breakpoint experiment and N=8–9 mice per genotype per treatment condition in the sociability investigation; *P<0.05, compared with the vehicle group or the vehicle within genotype; #P<0.05, compared with the WT vehicle control.
Sociability in NR1 Mice
Social withdrawal is a hallmark of SZ and NR1-KD mice display this response (Mohn et al, 1999). These mutants are deficient also in sociability and clozapine improves it (Park et al, 2016). WT and NR1-KD mice were tested in three phases: a non-social pairing with two identical stimuli, a pairing with social and non-social stimuli (social affiliation), and a pairing with the now familiar and a novel social stimulus (social preference). As a control, the duration of stimulus contacts was examined and no significant differences were observed among groups across test phases (Table 2)—indicating that any deficiencies in social behavior cannot be attributed to a failure to interact with the non-social/social stimuli. During the non-social pairing, no group showed any preference for one over the other identical stimulus (Figures 2e and f). By comparison, vehicle-treated WT mice preferred the novel social stimulus in the social affiliation test relative to NR1-KD mice—which showed no preference for either stimulus (P<0.001). Within genotype analyses revealed clozapine decreased WT performance compared with vehicle (P=0.010) (Figure 2e), whereas it augmented NR1-KD responses (P=0.001) (Figure 2f). Relative to vehicle, there was a tendency for 0.5 mg/kg JJ-3-45 (P=0.060) to reduce WT social affiliation, whereas it was increased in mutants given 0.5 mg/kg JJ-3-45 or 1 mg/kg JJ-5-34 as well as with 0.5 or 1 mg/kg JJ-3-42 or lorcaserin (P-values<0.006). In NR1-KD mice a dose-dependent increase in social affiliation was observed with JJ-5-34 (P=0.008). Analyses of responses between genotypes demonstrated that social affiliation was higher in WT than NR1-KD mice at both doses of JJ-5-34 (P-values<0.042). With the exception of 0.5 mg/kg JJ-5-34 responses, all other treatment conditions restored NR1-KD social affiliation to that of the WT vehicle controls.
In the social preference test, vehicle-treated WT mice preferred the novel over familiar social stimulus, whereas mutants displayed no preference for either stimulus (P<0.001) (Figures 2e and f). The within genotype comparisons revealed that relative to vehicle, clozapine decreased WT performance (P=0.001) (Figure 2e) with a tendency for it to facilitate social preference in NR1-KD mice (P=0.064) (Figure 2f). Compared with vehicle, social preference in WT controls was depressed with 1 mg/kg JJ-3-45 or JJ-3-42 as well as with 0.5 or 1 mg/kg JJ-5-34 and lorcaserin (P-values<0.024). In contrast, this behavior was increased in mutants with 0.5 mg/kg JJ-3-45 or JJ-3-42 or with 1 mg/kg JJ-5-34 and lorcaserin (P-values<0.050). A dose-dependent decrease in social preference was noted with JJ-3-42 (P=0.043) in WT mice (Figure 2e). Importantly, only 0.5 mg/kg JJ-3-42 restored social preference in NR1-KD mice to that of the WT vehicle controls (Figure 2e and f).
In summary, vehicle-treated NR1-KD mice were significantly impaired in both social affiliation and social preference relative to WT vehicle controls. However, all three JJ compounds and lorcaserin improved both social behaviors in mutants and decreased them in WT mice. Notably, only 0.5 mg/kg JJ-5-34 failed to restore social affiliation in NR1-KD mice to that of the WT vehicle controls. By comparison, only 0.5 mg/kg JJ-3-42 normalized social preference in mutants.
NORM in NR1 Mice
NORM examines recognition memory and general mnemonic function (Ennaceur and Delacour, 1988), and NR1-KD mice are deficient in NORM (Park et al, 2016). During training, WT and NR1-KD mice demonstrated no object preference between the two identical objects regardless of drug-dose assignment (Figure 3a and b). A comparison of preference scores between training and testing revealed that at testing WT mice failed to prefer the novel object when administered 5 mg/kg JJ-5-34, whereas NR1-KD animals were deficient when given the vehicle, 1 or 10 mg/kg JJ-3-42, 5 mg/kg JJ-5-34, or 0.5 mg/kg lorcaserin. Analyses of responses within genotype found that in WT mice all treatments—except 10 mg/kg JJ-3-42 and 1 mg/kg JJ-5-34—reduced NORM below that of the vehicle control (P-values<0.012). In contrast, in mutants 5 mg/kg JJ-3-42, as well as both doses of JJ-3-45, JJ-5-34, and lorcaserin augmented NORM over that of vehicle (P-values<0.024). In comparing responses to the WT vehicle control, 1 mg/kg JJ-3-45, 5 mg/kg JJ-3-42, and 1 mg/kg JJ-5-34 restored NORM in the NR1-KD mice. It is noteworthy that 1 mg/kg JJ-5-34 maintained NORM in WT mice, whereas this treatment normalized NORM in mutants.
The three JJ compounds and lorcaserin improved novel object recognition memory (NORM) in NR1-knockdown (NR1-KD) mice; however, JJ-3-45 was unable to restore reversal discrimination performance in MK-801-treated C57BL/6J mice. (a and b) Responses at training and at testing 24 h later in NORM with wild-type (WT) and NR1-KD mice. (a) The investigations were conducted with WT mice using different doses of JJ-3-45, JJ-3-42, JJ-5-34, and lorcaserin. (b) The same experiments were run with NR1-KD mice. A repeated-measures analysis of variance (RMANOVA) for JJ-3-45 within-subjects effect of time (F(1,46)=59.964, P<0.001), time by genotype (F(1,46)=8.521, P=0.005), time by treatment (F(2,46)=3.110, P=0.054), and time by genotype by treatment (F(2,46)=13.318, P<0.001) interactions; between-subjects effects of genotype (F(1,46)=12.403, P<0.001), genotype by treatment interaction (F(2,46)=12.136, P<0.001). The RMANOVA for JJ-3-42 within-subjects time effect (F(1,60)=73.254, P<0.001), time by genotype (F(1,60)=32.444, P<0.001) and time by genotype by treatment (F(3,60)=9.676, P<0.001) interactions; between-subjects genotype effect (F(1,60)=19.730, P<0.001), genotype by treatment interaction (F(3,60)=7.672, P<0.001). A RMANOVA for JJ-5-34 within-subjects effects of time (F(1,48)=40.152, P<0.001), time by genotype (F(1,48)=6.975, P=0.011), time by treatment (F(2,48)=4.892, P=0.012), and time by genotype by treatment (F(2,48)=8.493, P<0.001) interactions; between-subjects effects of genotype (F(1,48)=7.745, P=0.008), genotype by treatment interaction (F(2,48)=8.455, P=0.001). The RMANOVA for lorcaserin within-subjects main effects for time (F(1,26)=59.239, P<0.001), time by genotype (F(1,46)=16.858, P<0.001) and time by genotype by treatment (F(2,46)=15.199, P<0.001) interactions; between-subject tests for the genotype (F(1,46)=20.639, P<0.001), genotype by treatment interaction (F(2,46)=20.269, P<0.001). (c–f) Visual discrimination performance and reversal in C57BL/6J mice treated with vehicle, MK-801, or MK-801 plus JJ-3-45. (c) Baseline performance on the visual discrimination is presented as the numbers of correct responses, numbers of errors, and percent correct responses for groups given vehicle, MK-801, or JJ-3–45 plus MK-801. (d) Number of correct responses during the last two days of visual discrimination followed by reversal testing. A RMANOVA for the numbers of correct trials: within-subject effect of session (F(15,270)=50.264, P<0.001), session by treatment interaction (F(30,270)=4.828, P<0.001); between subject effect of treatment (F(2,18)=16.831, P<0.001). (e) Number of errors over the same course of testing. A RMANOVA for the number of errors: within-subjects effects for session (F(15,270)=60.559, P<0.001), session by treatment interaction (F(30,270)=2.664, P<0.001); between-subjects effect of treatment (F(2,18)=35.288, P<0.001). (f) Percent correct responses over the same course of testing. A RMANOVA for the percent of correct responses: within-subjects effects of session (F(15,270)=59.741, P<0.001), session by treatment interaction (F(30,270)=5.251, P<0.001); between-subjects effect of treatment (F(2,18)=17.346, P<0.001). N=8–10 mice per drug or compound per dose in NORM and N=5–10 mice per treatment in the reversal visual discrimination test; *P<0.05, compared with the vehicle group or the vehicle within genotype; #P<0.05, compared with the WT vehicle control.
As a control, the duration of object contacts was examined. Although the object interaction times in RMANOVA failed to reach statistical significance, the overall duration of contacts was enhanced in NR1-KD mice during both training and testing (Table 3). As a consequence, any NORM deficiencies in mutants cannot be attributed to a failure to engage the stimulus objects.
Reversal Learning with MK-801 in C57BL/6 Mice
Reversal learning is a test for plasticity or perseverative responding and it is used in a rodent test battery for SZ-like behaviors (Bussey et al, 2012). C57BL/6J mice were trained on a visual discrimination, administered vehicle, MK-801, or MK-801 plus JJ-3-45 during the latter part of the discrimination, and tested with reinforcement contingencies reversed. The preassigned groups did not differ at baseline in the simple visual discrimination (Figure 3c).
Relative to vehicle controls at reversal, the number of correct trials was reduced with MK-801 on trials 8 and 10–15 (P-values<0.048), whereas this metric was suppressed even further in the MK-801 plus JJ-3-45 group across trials 6–17 (P-values<0.033) (Figure 3d). Analyses of reversal performance according to the number of errors revealed increased errors in the MK-801 group on trials 3–11 and 13–15 (P-values<0.051), whereas errors were further potentiated across all trials in the MK-801 plus JJ-3-45 group (P-values<0.002) (Figure 3e). After contingency reversal, the percentages of correct responses were decreased in MK-801-treated mice on trials 8, 10–15, and 17 (Ps<0.054) and those in the MK-801 plus JJ-3-45 group were further depressed across trials 6–17 (P-values<0.029) (Figure 3f). Collectively, MK-801 alone and MK-801 plus JJ-3-45 reduced reversal performance below the vehicle control. Moreover, performance in the MK-801 plus JJ-3-45 group was further suppressed below that of MK-801 alone at a dose of JJ-3-45 that did not perturb the visual discrimination.
Cataleptic Potential of Compounds in C57BL/6 Mice
An important aspect for new generations of APDs is determining whether they possess motor side effects. In the horizontal bar test mice were evaluated before (baseline) and 60 min after treatment. Catalepsy at baseline was similar across all groups (range 0.0–1.0 s). Compared with vehicle, haloperidol produced significant catalepsy at 1 and 10 mg/kg (P-values<0.001), which were not significantly different from each other (Figure 4). Additionally, responses to 1 and 10 mg/kg haloperidol were significantly higher than those to lower doses of haloperidol and all other compounds (P-values<0.001). Thus, the novel 5-HT2C compounds and lorcaserin do not produce catalepsy at doses that elicit it with haloperidol.
The three JJ compounds and lorcaserin have low cataleptic potential in C57BL/6J mice compared with haloperidol in the horizontal bar test. Dose responses with haloperidol, JJ-3-45, JJ-3-42, JJ-5-34, and lorcaserin for catalepsy. An analysis of variance (ANOVA) between-subjects effects of treatment (F(20,175)=18.511, P<0.001). N=8–12 mice per compound or drug dose; *P<0.05, compared with the vehicle control; ‡P<0.05, 1 or 10 mg/kg haloperidol compared with all other doses and compounds.
Effects of JJ-3-42 and Lorcaserin on In Vivo Brain Neurotransmitter Release
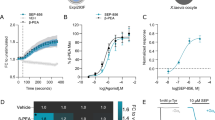
5-HT2C receptors are G-protein-coupled receptors expressed on GABAergic, glutamatergic, and dopaminergic neurons (Bubar et al, 2011; McCorvy and Roth, 2015; Nocjar et al, 2015). These receptors are present in cortical areas, hippocampus, ventral midbrain, striatum, NAc, hypothalamus, and amygdala (Jensen et al, 2010). Microdialysis studies were conducted to determine whether a 5-HT2C agonist or lorcaserin could influence neurotransmitter efflux in mouse infralimbic cortex or striatum. In infralimbic cortex, 10 mg/kg JJ-3-42 significantly reduced DA (P=0.040), 5-HT (P=0.014), DOPAC (P=0.003), and HVA (P=0.006), whereas 1 mg/kg lorcaserin decreased DA (P=0.021) and HVA (P=0.050) compared with vehicle (Figure 5a). In the striatum, only JJ-3-42 significantly decreased DA efflux (P=0.021) (Figure 5b). All other neurotransmitter levels were not significantly different among treatments.
JJ-3-42 and lorcaserin decrease dopamine efflux in infralimbic cortex, whereas only the JJ compound reduced it in the striatum of C57BL/6J mice. Baseline values were collected and then mice were injected (time 0) with vehicle, 10 mg/kg JJ-3-42, or 1 mg/kg lorcaserin and samples were collected over 180 min. Absolute baseline levels can be found in Supplementary Table S1. (a) Extracellular neurotransmitter and metabolite levels in the infralimbic cortex expressed as area under the curve (AUC) representing the mean values from 0 to 180 min. In infralimbic cortex, the following neurotransmitter efflux levels were significant by Kruskal–Wallis H tests: dopamine (DA) (P=0.035), serotonin (5-HT) (P=0.009), 3,4-dihydroxyphenylacetic acid (DOPAC) (P=0.002), and homovanillic acid (HVA) (P=0.016). (b) Efflux of neurotransmitter and metabolites in the striatum displayed as area under the curve (AUC). The Kruskal–Wallis H test discerned a significant effect for DA (P=0.042) and a trend for HVA (P=0.075). (c) Extracellular DA levels in the infralimbic cortex at baseline (−90, −60, −30, and 0 min) and subsequently (30, 60, 90, 120, 150, and 180 min) postinjection to the vehicle, JJ-3-42, and lorcaserin. A repeated-measures analysis of variance (RMANOVA) for responses at baseline (−90 to 0 min) to 180 min: within-subjects effects of time (F(6,114)=3.927, P<0.001), time by treatment interaction (F(12.114)=1.943, P=0.036); between-subjects effects of treatment (F(2,19)=5.458, P=0.013). (d) Extracellular DA levels in the striatum during baseline and following exposure to the vehicle, JJ-3-42, and lorcaserin. Kruskal–Wallis H tests for DA at 60, 90, and 150 min (P-values<0.054). N=7–9 mice per treatment; *P<0.05, compared with the vehicle group; xP<0.05, lorcaserin vs JJ-3-42.
Responses to JJ-3-42 and lorcaserin on cortical and striatal DA efflux over time are displayed in Figures 5c and d, respectively. In these brain regions, no significant pre-treatment effects were observed at baseline (−90, −60, −30, and 0 min). At 90 min postinjection, infralimbic cortex extracellular DA levels were decreased with lorcaserin (P=0.029) and they were even more suppressed with JJ-3-42 relative to the vehicle control (P=0.004) (Figure 5c). DA levels were still depressed at 120 min with JJ-3-42 (P-values<0.031), whereas levels recovered to those of the vehicle with lorcaserin. In contrast, striatal DA levels were reduced at 60, 90, and 150 min postinjection with JJ-3-42 (P-values<0.029), but not with lorcaserin (Figure 5d). Collectively, JJ-3-42 exerts more marked effects than lorcaserin on DA efflux in infralimbic cortex and only JJ-3-42 reduced DA in the striatum.
Discussion
New medications to treat positive, negative, and cognitive symptoms of SZ that have superior efficacy and fewer side effects than typical and atypical APDs are crucial to achieve better outcomes in various psychotic disorders. In this regard, compounds that can modulate DA release such as 5-HT2C-selective agonists (Cheng et al, 2015, 2016) show some promise. In the present study, we provide behavioral profiles of three novel selective 5-HT2C agonists in models of SZ-like behaviors and have analyzed one of the compounds and lorcaserin on neurotransmitter efflux. It should be emphasized that our results are relevant to SZ because clinical trials were completed in 2014 for a 5-HT2C-selective drug (https://clinicaltrials.gov/ct2/show/results/NCT00563706?sect=X543016#limit).
JJ-3-42 and JJ-3-45 are structurally unique compounds that show good potency and excellent selectivity for the 5-HT2C receptor (Cheng et al, 2015, 2016). Both compounds are 5-HT2C agonists (EC50s of 4.2 and 3.4 nM for JJ-3-42 and JJ-3-45, respectively), with ~100-fold selectivity against 5-HT2A as partial agonists (Emax=56 and 76% for JJ-3-42 and JJ-3-45, respectively). Most importantly, these compounds show no significant activation of 5-HT2B receptors at concentrations up to 10 μM, whereas lorcaserin is a full agonist with moderate potency in our assay (EC50=478 nM, Emax=92%). JJ-5-34 is comprised of a benzofuran scaffold and it activates the 5-HT2C receptor with an EC50 of 23 nM (Emax=102%). It is a very weak 5-HT2B agonist (EC50=433 nM, Emax=19%), with no activation of 5-HT2A receptors up to 10 μM (results to be published). Besides their excellent pharmacological profiles, in CD-1 mice all three compounds have excellent brain penetration properties, with brain/plasma concentration ratios >5 at all time-points tested (data not shown). These high brain concentrations indicate that the JJ compounds may be actively transported into the brain, which is ideal for CNS-targeted therapeutics. Despite their excellent profile, the compounds may have some activities that were not assayed. Regardless, all three JJ compounds represent excellent pharmacological tools for exploring the therapeutic effects of 5-HT2C-selective agonists in SZ models and they represent potential 5-HT2C receptor drug candidates for further development.
Acute AMPH-induced hyperactivity is used as an animal model for acute psychosis as AMPH administration to SZ patients exacerbates the florid symptomatology (Angrist et al, 1980). All three JJ compounds dose-dependently reduced AMPH-stimulated hyperlocomotion. With the exception of 30 mg/kg JJ-3-45, these effects did not affect baseline activity. JJ-3-42 and JJ-5-34 depressed AMPH-induced hyperactivity to levels that were statistically indistinguishable from vehicle controls. JJ-3-45 was not as efficacious since activity with 20 mg/kg was still significantly higher than vehicle, whereas 30 mg/kg significantly depressed baseline locomotion. By comparison, lorcaserin had a stimulatory effect on baseline activity at the lowest dose and the reduction in AMPH-induced hyperlocomotion was not dose-dependent—suggesting a complex mechanism of action (see Devroye et al, 2015).
Atypical APDs exert their actions through antagonism of D2, inverse agonism at 5-HT2A, and various actions at 5-H1A, 5-HT6, and 5-HT7 receptors—depending on the drug administered (Meltzer and Huang, 2008). Typical APDs act primarily through D2 receptor blockade. The novel JJ compounds do not block DA receptors (Cheng et al, 2016). However, they reduce DA receptor stimulation by decreasing DA release and by acting on 5-HT2C receptors on GABAergic neurons in the substantia nigra and VTA (Gobert et al, 2000). This latter mode of action may underlie the lack of significant cataleptic potential of JJ compounds. Although lorcaserin produced catalepsy over baseline; this response was not significantly higher compared with that to vehicle. With regard to JJ compounds, it should be emphasized that at 20 mg/kg, each compound significantly antagonized open-field AMPH-stimulated hyperactivity while producing no catalepsy in the horizontal bar test. By comparison, typical APDs with relatively selective D2 antagonism as well as atypical APDs produce similar reductions in AMPH-induced hyperlocomotion at doses that yield significant depressions in baseline activity or produce catalepsy (Schaefer and Michael, 1984).
PPI is disrupted in acute psychosis (Meincke et al, 2004) and it is perturbed in rodents by direct and indirect DA agonists (Geyer and Moghaddam, 2002). Both typical and atypical APDs reverse these effects. JJ-3-42 and JJ-5-34 at 1 to 4 mg/kg normalized AMPH-disrupted PPI to the vehicle control; JJ-3-45 and lorcaserin at 0.5 mg/kg were more efficacious. These doses were much lower than those needed to affect baseline open field locomotion or antagonize the AMPH-induced hyperactivity. By comparison, the doses of conventional APDs used to normalize AMPH-disrupted PPI (Brody et al, 2004; Feifel et al, 1999; Swerdlow et al, 2005) are in the range of doses that can affect baseline locomotion or block AMPH-induced hyperlocomotion, while the atypical APD clozapine restores PPI at 20 mg/kg, it does not normalize locomotion in rats (Cáceda et al, 2005). Thus, JJ compounds display superior efficacy in normalizing PPI in the AMPH model and they may possess a broader therapeutic index than typical APDs for treating acute psychosis.
Some negative symptoms of SZ amenable to modeling in animals are motivational impairment and diminished social drive (Kirkpatrick et al, 2000). Second-generation APDs partially ameliorate negative symptoms (Leucht et al, 1999) and D2/D3 antagonists may cause secondary negative symptoms (Heinz et al, 1998). To assess motivation, we used a progressive ratio schedule of food reinforcement. Breakpoint in rodents is correlated with the magnitude of sucrose reward (Young and Markou, 2015) and with exertion of effort (Salamone and Correa, 2012). Effort-based tasks have been proposed for modeling motivational abnormalities in SZ based on preserved hedonic responses to primary rewards (Young and Markou, 2015). We found all three JJ compounds and lorcaserin reduced breakpoints in the same dose ranges that were effective in antagonizing AMPH-disrupted PPI. In this respect, the selective 5-HT2C ligands behaved similar to typical APDs (Salamone et al, 1991). Hence, these compounds may not have the potential to reverse motivation-related deficits in SZ, which continue to be a challenging area in the management of SZ.
Currently, the nature of impaired social interactions in SZ is obscure where they may have complex relationships with positive and negative symptoms, reflect social anxiety (Millan and Bales, 2013), or perturbed executive function (Meltzer et al, 1996). Hence, social behavior in animal models of SZ should be interpreted with caution. Nevertheless, a commonly used procedure with rodents is the sociability test (Moy et al, 2004). This test examines social affiliation, which may represent motivation to spend time with a conspecific vs an inanimate object and social preference, which denotes an ability to differentiate between familiar and novel social stimuli. We applied this test to NR1-KD mice, which are considered to mimic the hypothesized NMDAR hypofunction in SZ and are hyperactive in the open field, impaired in PPI, and deficient in sociability (Mohn et al, 1999; Park et al, 2016).
All three selective 5-HT2C agonists and lorcaserin significantly improved social affiliation in NR1-KD mice in a dose range similar to clozapine. Although clozapine increased social affiliation in mutants to that of WT vehicle controls, their overall levels of social behavior were lower. The results in NR1-KD mice appear more marked than in SZ patients where clozapine exerts only modest effects on psychosocial functioning as measured with the Quality of Life Scale and other questionnaires that assess social functioning and activities of daily living (Swartz et al, 2007; Guo et al, 2012). In a previous study, diazepam improved the abilities of NR1-KD mice to prefer social over non-social stimuli (Milenkovic et al, 2014). It remains to be seen whether the selective 5-HT2C agonists possess anxiolytic properties like diazepam. In this respect, an HTR2c polymorphism has been associated with enhanced accumbal DA release during pain stress (Mickey et al, 2012). Hence, by acting on the mesolimbic DA system, selective 5-HT2C agonists may possess anxiolytic actions and ameliorate stress responses.
The NR1-KD mice were impaired also in social preference. Since there was a 1–2 min delay between the social affiliation and social preference tests, this deficit is unlikely to represent impaired memory function. Clozapine failed to improve social preference in NR1-KD subjects; however, other doses may be more effective. By comparison, lower doses of JJ-3-45 and JJ-3-42 and higher doses of JJ-5-34 and lorcaserin improved social preference in these mutants. While the underlying mechanism requires additional investigation, one process relevant to SZ symptomatology may be deficient encoding of information. For instance, a discrimination of social novelty requires selective attention and this can be disrupted by acute or neonatal phencyclidine treatment that is sensitive to clozapine (Terranova et al, 2005). While attention has not been assessed in NR1-KD mice, blockade of NMDARs in frontal cortex reduces accuracy in the 5-choice serial reaction-time task in rodents due to ‘distractability’ (Carli and Invernizzi, 2014). Thus, it is possible that 5-HT2C agonists may affect selective attention independent of memory processes in NR1-KD mice.
In addition to various psychosocial symptoms, non-verbal memory deficits in SZ are well documented, especially for incidental visual memory (Seidman et al, 2003). A deficit in a subtype of long-term memory termed episodic memory is considered a core deficit in SZ (Clare et al, 1993) and the NORM task requires intact visual memory for a previously encountered item (see Ennaceur and Delacour, 1988). JJ-3-45, JJ-5-34, and lorcaserin reversed the NR1-KD impairment in NORM at 1 mg/kg. Parenthetically, this same dose restored social affiliation in these mutants. By comparison, JJ-3-42 normalized NORM in NR1-KD mice at 5 mg/kg. Higher doses of all compounds were not efficacious—suggesting an optimum for stimulation of 5-HT2C receptors in this task.
Another cognitive symptom of SZ pertains to an inability to suppress a previous response when it becomes irrelevant and this deficiency has been attributed to reduced sensitivity to negative feedback (Leeson et al, 2009). This condition, perseveration, can be modeled by reversing the contingencies of a simple visual discrimination (Bussey et al, 2012). With this paradigm, we examined effects of chronically administering JJ-3-45 to mice given the NMDAR antagonist MK-801, as another model for cognitive impairment in SZ (van der Meulen et al, 2003). We empirically selected a dose of MK-801 that did not perturb visual discrimination performance but significantly retarded both the learning of a new contingency and the extinction of the prepotent response to the irrelevant stimulus. Here, mice treated with MK-801 made more errors after stimulus reversal and only by the end of 14 days of testing did they approach the accuracy of vehicle controls. Reversal learning depends upon the 5-HT system and Alsiö et al (2015) found that systemic 5-HT2C antagonism alleviated early (ie, occurring before achieving 50% correct responses) perseverative errors. Our results with the agonist JJ-3-45 agree with this finding where this compound potentiated the effects of MK-801-induced perseveration as demonstrated by a higher proportion of errors from the beginning of reversal through the final sessions, compared with MK-801 treatment alone. Thus, selective 5-HT2C agonists may have the potential to exacerbate this executive-function deficit in SZ.
5-HT2C receptors are expressed on GABAergic, glutamatergic, and dopaminergic neurons (Bubar et al, 2011; McCorvy and Roth, 2015; Nocjar et al, 2015). 5-HT2C agonists (eg, WAY 163909, CP-809101, and vabicaserin) have been proposed to offer potential antipsychotic efficacy without possessing the traditional side-effects associated with current APDs (Dunlop et al, 2006; Marquis et al, 2007; Siuciak et al, 2007; Aloyo et al, 2009; Jensen et al, 2010; Rosenzweig-Lipson et al, 2012; Liu et al, 2014; Cheng et al, 2015). These agonists can reduce basal firing rates and bursting activity of DA neurons and exert an excitatory effect on many GABAergic neurons in the VTA (Invernizzi et al, 2007; Di Giovanni et al, 2000), suggesting a possible mechanism of action for these agonists. In a microdialysis study with rats, 5-HT2C agonists (ie, WAY 163909, Ro-61-0175, and MK 212) decreased DA in the medial prefrontal cortex and NAc, while increasing ACh efflux in the prefrontal cortex and hippocampus (Millan et al, 1998; Nair and Gudelsky, 2004; Marquis et al, 2007). We found that 10 mg/kg JJ-3-42 and 1 mg/kg lorcaserin reduced infralimbic DA efflux, while only JJ-3-42 successfully decreased it in striatum—suggesting that their mechanisms of actions may be different. Since the DA hypothesis of SZ postulates that extracellular DA levels are low in cortex and high in striatum (see Weinstein et al, 2016), it is possible that 5-HT2c agonists may exacerbate some symptoms of SZ by further reducing cortical DA. Nevertheless, these findings support the hypothesis that 5-HT2C receptors in the NAc and VTA negatively regulate DA efflux from dopaminergic neurons (O’Neil, 2010). Our findings suggest that JJ-3-42 may stimulate 5-HT2C receptors in the NAc or VTA, and thereby inhibit dopaminergic neurons that project to the frontal cortex and striatum, respectively. Other APD-like mechanisms of 5-HT2C agonists may include modification of signaling pathways and trafficking, as well as RNA-editing changes that have been associated with SZ (Rosenzweig-Lipson et al, 2012).
SZ is a complex neuropsychiatric disorder and responses to APDs are heterogeneous. Despite multiple actions at D2 and other receptors, most APDs are efficacious in treating positive symptoms but are less robust for treating negative and cognitive symptoms (Leucht et al, 2009; Miyamoto et al, 2012). In the present studies, we demonstrate that the 5-HT2C agonists—JJ-3-45, JJ-3-42, and JJ-5-34—are mostly efficacious in ameliorating a broad range of SZ-like behaviors in mice. Importantly, these compounds show efficacy in mice with persistent NMDAR hypofunctioning and in the hyperdopaminergic AMPH model of SZ. While we do not know whether 5-HT2C agonists will be efficacious in treating patients, the ability of these ligands to modulate dopaminergic activities in the frontal cortex and striatum may provide a unique opportunity to develop drugs with fewer side effects, greater therapeutic selectivity, and enhanced efficacy for treating SZ and related disorders than current APDs.
Funding and disclosure
The authors declare no conflict of interest.
References
Abdallah L, Bonasera SJ, Hopf FW, O'Dell L, Giorgetti M, Jongsma M et al (2009). Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci 29: 8156–8165.
Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA (2005). Modulation of dopamine release by striatal 5-HT2C receptors. Synapse 55: 242–251.
Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA (2009). Current status of inverse agonism at serotonin 2A (5-HT2A and 5-HT2C receptors. Pharmacol Ther 121: 160–173.
Alsiö J, Nilsson SR, Gastambide F, Wang RA, Dam SA, Mar AC et al (2015). The role of 5-HT2C receptors in touchscreen visual reversal learning in the rat: a cross-site study. Psychopharmacology (Berl) 232: 4017–4031.
Angrist B, Rotrosen J, Gershon S (1980). Differential effects of amphetamine and neuroleptics on negative vs. positive symptoms in schizophrenia. Psychopharmacology (Berl) 72: 17–19.
Arciniegas DB, Topkoff JL, Held K, Frey L (2001). Psychosis due to neurologic conditions. Curr Treat Options Neurol 3: 347–366.
Brody SA, Conquet F, Geyer MA (2004). Effect of antipsychotic treatment on the prepulse inhibition deficit of mGluR5 knockout mice. Psychopharmacology 172:187–195..
Bubar MJ, Stutz SJ, Cunningham KA (2011). 5-HT2C receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One 6: e20508.
Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J et al (2012). New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62: 1191–1203.
Cáceda R, Kinkead N, Owens MJ, Nemeroff CB (2005). Virally mediated increased neurotensin 1 receptor in the nucleus accumbens decreases behavioral effects of mesolimbic system activation. J Neurosci 25: 11748–11756.
Carli M, Invernizzi RW (2014). Serotoninergic and dopaminergic modulation of cortico-striatal circuit in executive and attention deficits induced by NMDA receptor hypofunction in the 5-choice serial reaction time task. Front Neural Circuits 8: 58.
Cheng J, Giguère PM, Onajole OK, Lv W, Gaisin A, Gunosewoyo H et al (2015). Optimization of 2-phenylcyclopropylmethylamines as selective serotonin 2C receptor agonists and their evaluation as potential antipsychotic agents. J Med Chem 58: 1992–2002.
Cheng J, Giguère PM, Schmerberg CM, Pogorelov VM, Rodriguiz RM, Huang XP et al (2016). Further advances in optimizing (2-phenylcyclopropyl)methylamines as novel serotonin 2C agonists: effects on hyperlocomotion, prepulse inhibition, and cognition models. J Med Chem 59: 578–591.
Clare L, McKenna PJ, Mortimer AM, Baddeley AD (1993). Memory in schizophrenia: What is impaired and what is preserved? Neuropsychologia 31: 1225–1241.
Conn PJ, Sanders-Bush E, Hoffman BJ, Hartig PR (1986). A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover. Proc Natl Acad Sci USA 83: 4086–4088.
Désaméricq G, Schurhoff F, Meary A, Szöke A, Macquin-Mavier I, Bachoud-Lévi AC et al (2014). Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol 70: 127–134.
Devroye C, Cathala A, Maitre M, Piazza PV, Abrous DN, Revest JM et al (2015). Serotonin 2C receptor stimulation inhibits cocaine-induced Fos expression and DARPP-32 phosphorylation in the rat striatum independently of dopamine outflow. Neuropharmacology 89: 375–381.
Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E (2000). Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin2C/2B receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse 35: 53–61.
Dunlop J, Marquis KL, Lim HK, Leung L, Kao J, Cheesman C et al (2006). Pharmacological profile of the 5-HT2C receptor agonist WAY-163909; therapeutic potential in multiple indications. CNS Drug Rev 12: 167–177.
Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF (1997). Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol 384: 233–247.
Ennaceur A, Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59.
Feifel D, Reza T, Robeck S (1999). Antipsychotic potential of CCK-based treatments: an assessment using the prepulse inhibition model of psychosis. Neuropsychopharmacology 20:141–149.
Geyer MA, Moghaddam B (2002). Animal models relevant to schizophrenia disorders. In: David KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins: Philadelphia, PA. pp 689–701.
Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP et al (2000). Serotonin2C receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse 36: 205–221.
Grottick AJ, Fletcher PJ, Higgins GA (2000). Studies to investigate the role of 5-HT2C receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther 295: 1183–1191.
Guo X, Zhang Z, Zhai J, Fang M, Hu M, Wu R et al. Early-stage Schizophrenia Outcome Study Investigators (2012). Effects of antipsychotic medications on quality of life and psychosocial functioning in patients with early-stage schizophrenia: 1-year follow-up naturalistic study. Compr Psychiatry 53: 1006–1012.
Heinz A, Knable MB, Coppola R, Gorey JG, Jones DW, Lee KS et al (1998). Psychomotor slowing, negative symptoms and dopamine receptor availability—an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophr Res 31: 19–26.
Invernizzi RW, Pierucci M, Calcagno E, Di Giovanni G, Di Matteo V, Benigno A et al (2007). Selective activation of 5-HT2C receptors stimulates GABA-ergic function in the rat substantia nigra pars reticulata: a combined in vivo electrophysiological and neurochemical study. Neuroscience 144: 1523–1535.
Jensen NH, Cremers TI, Sotty F (2010). Therapeutic potential of 5-HT2C receptor ligands. Scientific World J 10: 1870–1885.
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M et al (2010). Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67: 231–239.
Kirkpatrick B, Kopelowicz A, Buchanan RW, Carpenter WT Jr (2000). Assessing the efficacy of treatments for the deficit syndrome of schizophrenia. Neuropsychopharmacology 22: 303–310.
Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS et al (1999). NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry 7: 125–143.
Landqvist-Waldö M, Gustafson L, Passant U, Englund E (2015). Psychotic symptoms in frontotemporal dementia: a diagnostic dilemma? Int Psychogeriatr 27: 531–539.
Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR et al (2009). Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry 66: 586–593.
Leucht S, Pitschel-Walz G, Abraham D, Kissling W (1999). Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res 35: 51–68.
Leucht S, Arbter D, Engel RR, Kissling W, Davis JM (2009). How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 14: 429–447.
Liu J, Ogden A, Comery TA, Spiros A, Roberts P, Geerts H (2014). Prediction of efficacy of vabicaserin, a 5-HT2C agonist, for the treatment of schizophrenia using a quantitative systems pharmacology model. CPT Pharmacometrics Syst Pharmacol 3: e111.
López-Gil X, Artigas F, Adell A (2010). Unravelling monoamine receptors involved in the action of typical and atypical antipsychotics on glutamatergic and serotonergic transmission in prefrontal cortex. Curr Pharm Des 16: 502–515.
Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA et al (2007). WAY-163909[(7bR,10aR-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino [6,7,1hi]indole]: a novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 320: 486–496.
McCorvy JD, Roth BL (2015). Structure and function of serotonin G-protein coupled receptors. Pharmacol Ther 150: 129–142.
Meincke U, Mörth D, Voss T, Thelen B, Geyer MA, Gouzoulis-Mayfrank E (2004). Prepulse inhibition of the acoustically evoked startle reflex in patients with an acute schizophrenic psychosis—a longitudinal study. Eur Arch Psychiatry Clin Neurosci 254: 415–421.
Meltzer HY (2015). Pharmacotherapy of cognition in schizophrenia. Curr Opin Behav Sci 4: 115–121.
Meltzer HY, Matsubara S, Lee JC (1989). Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2, and serotonin2 pKi values. J Pharmacol Exp Ther 251: 238–246.
Meltzer HY, Thompson PA, Lee MA, Ranjan R (1996). Neuropsychologic deficits in schizophrenia: relation to social function and effect of antipsychotic drug treatment. Neuropsychopharmacology 14 (Suppl): 27S–33S.
Meltzer HY, Huang M (2008). In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res 172: 177–197.
Mickey BJ, Sanford BJ, Love TM, Shen PH, Hodgkinson CA, Stohler CS et al (2012). Striatal dopamine release and genetic variation of the serotonin 2C receptor in humans. J Neurosci 32: 9344–9350.
Milenkovic M, Mielnik CA, Ramsey AJ (2014). NMDA receptor-deficient mice display sexual dimorphism in the onset and severity of behavioural abnormalities. Genes Brain Behav 13: 850–862.
Millan MJ, Dekeyne A, Gobert A (1998). Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology 37: 953–955.
Millan MJ, Bales KL (2013). Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev 37: 2166–2180.
Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005). Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10: 79–104.
Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry 17: 1206–1227.
Mohn AR, Gainetdinov RR, Caron MG, Koller BH (1999). Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98: 427–436.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR et al (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3: 287–302.
Nair SG, Gudelsky GA (2004). Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse 53: 202–207.
Navailles S, De Deurwaerdère P, Porras G, Spampinato U (2004). In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology 29: 319–326.
Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA (2015). Serotonin-2c and -2a receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience 297: 22–37.
O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J et al (2012). Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring, MD) 20: 1426–1436.
O’Neil R (2010). Role of the 5HT2C receptor in regulation of metabolism and mesolimbic dopamine. Vanderbilt Rev 2: 19–24.
Park SM, Chen M, Schmerberg C, Dulman R, Rodriguiz RM, Caron MG et al (2016). Effects of β-arrestin-biased dopamine D2 receptor ligands on schizophrenia-like behavior in hypoglutamatergic mice. Neuropsychopharmacology 41: 704–715.
Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdère P, Caccia S et al (2002). 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology 26: 311–324.
Rosenzweig-Lipson S, Comery TA, Marquis KL, Gross J, Dunlop J (2012). 5-HT2C agonists as therapeutics for the treatment of schizophrenia. Handb Exp Pharmacol 213: 147–165.
Roth BL, Scheffler DJ, Kroeze WK (2004). Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3: 353–359.
Salamone JD, Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76: 270–285.
Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K (1991). Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology 4: 515–521.
Schaefer GJ, Michael RP (1984). Drug interactions on spontaneous locomotor activity in rats. Neuroleptics and amphetamine-induced hyperactivity. Neuropharmacology 23: 909–914.
Seidman LJ, Lanca M, Kremen WS, Faraone SV, Tsuang MT (2003). Organizational and visual memory deficits in schizophrenia and bipolar psychoses using the Rey–Osterrieth complex figure: effects of duration of illness. J Clin Exp Neuropsychol 25: 949–964.
Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S et al (2010). Behavioral modification and lorcaserin for overweight and obesity management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 363: 245–256.
Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P et al (2007). CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52: 279–290.
Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G, Rosenheck RA et al (2007). CATIE investigators. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry 164: 428–436.
Swerdlow NR, Shoemaker JM, Bongiovanni MJ, Neary AC, Tochen LS, Saint Marie RL (2005). Reduced startle gating after D1 blockade: effects of concurrent D2 blockade. Pharmacol Biochem Behav 82: 293–299..
Terranova JP, Chabot C, Barnouin MC, Perrault G, Depoortere R, Griebel G et al (2005). SSR181507, a dopamine D2 receptor antagonist and 5-HT1A receptor agonist, alleviates disturbances of novelty discrimination in a social context in rats, a putative model of selective attention deficit. Psychopharmacology (Berl) 181: 134–144.
van der Meulen JA, Bilbija L, Joosten RN, de Bruin JP, Feenstra MG (2003). The NMDA-receptor antagonist MK-801 selectively disrupts reversal learning in rats. Neuroreport 14: 2225–2228.
Weinstein JJ, Muhammad OC, Slifstein M, Kegles LS, Moore H, Abi-Darham A (2017). Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiat in press 11: 551–568.
Wigman JTW, van Nierop M, Vollebergh WAM, Lieb R, Beesdo-Baum K, Wittchen H-U et al (2012). Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity—Implications for diagnosis and ultra-high risk research. Schizophr Bull 38: 247–257.
Young JW, Markou A (2015). Translational rodent paradigms to investigate neuromechanisms underlying behaviors relevant to amotivation and altered reward processing in schizophrenia. Schizophr Bull 41: 1024–1034.
Acknowledgements
We thank Shaina Gong, Vineet Nadkarni, Paul Skiba, Christopher Means, Liza Stephanz, Theodore Rhodes, and Aran Marati for assisting with some of the behavioral testing and Jiechun Zhou for genotyping and maintaining the mice. Some of the behavioral experiments were conducted with equipment and software purchased with a North Carolina Biotechnology Center grant. We also received the NR1 mice from Dr Amy J Ramsey (University of Toronto, Toronto, ON, Canada) and they were further backcrossed for more than 10 generations to C57BL/6 and 129X1/Sv mice as separate lines. We have no competing interests in relation to the work described herein. Dr Roth has received compensation as Deputy Editor of the Journal of Clinical Investigation and as consultant over the past 12 months from Pfizer Pharmaceuticals. Dr Roth has received research support from Merck Pharmaceuticals and Asubio Pharmaceuticals over the past 12 months. Dr Wetsel has received research support over the past 12 months from Rugen Holdings (Cayman) Limited. Dr Meltzer has received funding from Sumitomo Dainippon, Sunovion, Janssen Pharmaceuticals, Auspex, Lundbeck, and the Weisman Family Foundation. Drs Meltzer, Roth, Kozikowski, and Wetsel receive NIH funding. We are indebted to the NIMH (Grant No. R01MH99993) for financial support for this work. Some work in this manuscript from Drs Roth and Wetsel was supported by U19MH082441.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Pogorelov, V., Rodriguiz, R., Cheng, J. et al. 5-HT2C Agonists Modulate Schizophrenia-Like Behaviors in Mice. Neuropsychopharmacol 42, 2163–2177 (2017). https://doi.org/10.1038/npp.2017.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2017.52
This article is cited by
-
Pharmacological Targets and Mechanisms of Action of Antipsychotic Substances in the Context of the Neurochemical Theory of the Pathogenesis of Schizophrenia
Neuroscience and Behavioral Physiology (2022)
-
Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity
Nature (2022)
-
G protein-coupled receptors: structure- and function-based drug discovery
Signal Transduction and Targeted Therapy (2021)
-
Effects of the Interaction of the ANKK1/DRD2 TaqIA and HTR2C Cys23Ser Polymorphisms on Approach Motivation in Schizophrenia Patients and Healthy People
Neuroscience and Behavioral Physiology (2019)