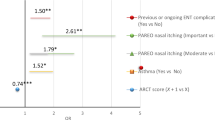
Abstract
Background:
Seasonal allergic rhinitis is typically poorly managed, particularly in adolescents, in whom it is responsible for considerable morbidity. Our previous work has demonstrated that if poorly controlled this can impair educational performance.
Aim:
The primary aim of this trial was to assess the impact of a primary care–based professional training intervention on clinical outcomes in adolescents with seasonal allergic rhinitis.
Methods:
Cluster trial in which UK general practice staff were randomised to a short, intensive workshop on the evidence-based management of seasonal allergic rhinitis. The primary outcome measure was the change in the validated Rhinoconjunctivitis Quality of Life Questionnaire with Standardized Activities (RQLQ(S)) score between baseline and 6 weeks post intervention (minimal clinically important difference=0.5). Secondary outcome measures of interest included health-care professionals’ knowledge and confidence in managing seasonal allergic rhinitis, number of seasonal allergic rhinitis-related consultations, relevant treatments prescribed and symptom scores.
Results:
Thirty-eight general practices were randomised (20 in the intervention arm) and 246 patients (50.2% males, mean age 15 years) were included in the primary outcome analysis. Health-care professionals’ knowledge and confidence of the clinical management of seasonal allergic rhinitis improved. This did not, however, result in clinically or statistically significant improvements in RQLQ(S): −0.15, (95% confidence interval, −0.5 to +0.2). There were no differences in consultation frequency, treatments issued for seasonal allergic rhinitis or symptom scores.
Conclusions:
Although associated with increases in professionals’ self-assessed confidence and understanding of seasonal allergic rhinitis management, this intensive training workshop did not translate into improvements in adolescents’ disease-specific quality of life or a reduction in rhinitis symptoms.
Similar content being viewed by others
Introduction
Seasonal allergic rhinitis (also known as intermittent allergic rhinitis) is a very common condition in adolescence, with national1 and international2–4 studies suggesting that up to 40% of young people may be affected. It can be responsible for considerable morbidity in its own right; research focusing on children and adolescents with seasonal allergic rhinitis has identified particular problems with schoolwork,5 exam performance6 as well as loss of sleep and reduced ability to concentrate.7,8 As a consequence, seasonal allergic rhinitis poses a substantial and increasing economic burden on health-care systems and the society at large.9 American estimates have, for example, suggested that health-care expenditure associated with allergic rhinitis has doubled since 2000, increasing to more than $11 billion.10 It is now better recognised that many people with seasonal allergic rhinitis also have coexistent asthma and this, together with a greater appreciation of their shared pathophysiology, has led the World Health Organization to promote the idea of ‘one airway, one disease’—i.e., that allergic rhinitis and asthma are different manifestations of the same disease.
Considerable time and resources are expended on educational interventions for health-care professionals, both in the United Kingdom and internationally, but despite these investments their impact on patient outcomes is still unknown. This is because such interventions are rarely evaluated beyond simple measures of satisfaction completed by the attendee, with little or no assessment of whether there is any impact on clinical practice or benefits to patients. Any intervention requiring time and/or financial commitment should be subject to the same rigorous evaluation as any other pharmacological or non-pharmacological intervention, and this is particularly true in financially constrained times. More specifically, the management of people with seasonal allergic rhinitis (and allergic rhinitis more generally) has been highlighted as being suboptimal by a number of studies11,12 and guidelines,8 with these inadequacies resulting in substantial—potentially avoidable—morbidity and cost. The overwhelming majority of people with seasonal allergic rhinitis are managed in the community;13 hence, this sector needs to be the focus of any attempt to improve the quality of care and outcomes.2
In an earlier multicentre randomised controlled trial,14 it was demonstrated that a part-time 6-month diploma-level allergy course was acceptable to attending primary health-care professionals, led to changes in relevant process measures (such as knowledge and confidence) and, importantly, translated into significant improvements in validated measures of disease-specific quality of life in adults. Although effective, many primary health-care professionals found this length of training difficult to incorporate into their practice, which raises important questions about the wider generalisability and sustainability of this approach. Following the approach advocated by the Medical Research Council’s Framework for Complex Interventions,15,16 we used our experiences from this earlier trial and related work on the training needs of primary care professionals in the context of managing seasonal allergic rhinitis to inform the development of the current intervention.12,17,18 In seeking to mirror the ways in which the majority of UK health-care professionals receive their continuing professional education, we developed an intensive, evidence-based 1-day educational training intervention for primary care professionals. We then sought to evaluate the effectiveness of this training intervention for primary care–based health-care professionals on adolescent disease-specific quality of life.
Materials and Methods
Overview of study design
We conducted a cluster randomised controlled trial of an educational intervention, which involved randomising general practice staff to receive either evidence-based allergy training or to the control arm in which practices received written guidance on the evidence-based management of seasonal allergic rhinitis.19 We chose a cluster randomised controlled trial, as a parallel group trial design would have resulted in a high risk of contamination. In keeping with recommended practice, our trial protocol and detailed analysis plan were reported before the closure of the trial;20 any deviations from the methods described in the protocol are detailed below.
Setting and participants
This trial took place during the summers of 2009 and 2010 in 38 general practices within the recruitment areas of the Scottish Primary Care Research Network and England’s Northern and Yorkshire Research Network. Scottish Primary Care Research Network and England’s Northern and Yorkshire Research Network invited general practices to take part by email and letter. Each consenting practice was asked to nominate a member of their team to participate who had not received postgraduate allergy training in the previous 12 months. All patients aged 12–18 years with current seasonal allergic rhinitis were eligible to participate. We defined current seasonal allergic rhinitis by the presence of a documented clinician diagnosis in the patient’s electronic health record and any evidence of treatment(s) used for seasonal allergic rhinitis in the last 2 years.21 Patients who fulfilled these criteria were invited to take part in the trial via a letter from the practice, which included a participant information sheet, consent form and reply envelope for return directly to the research team.
Randomisation and blinding
Practices were stratified on the basis of each of six regions: NHS Lothian; NHS South of Tyne and Wear; NHS North of Tyne; NHS County Durham; NHS North Yorkshire and York; and NHS Leeds; for regions where there were more than two clusters, a centrally administered minimisation scheme22 based on practice size and deprivation score was applied. Patients were masked to the allocation; however, it was not possible to mask the general practices as the intervention was attendance at a training workshop.
Intervention
Education for Health (http://www.educationforhealth.org.uk/) is one of UK’s leading independent national training organisations offering accredited allergy training for professionals. We worked with Education for Health to develop an intensive 1-day evidence-based workshop, which was customised to meeting the needs of adolescents (see Box 1). The intervention consisted of an intensive study day focused on the diagnosis and management of young people with seasonal allergic rhinitis. The programme began with an assessment of the participant’s current allergy knowledge, followed by the presentation of a case study, a discussion about the importance of getting the diagnosis of allergy correct and reasons for treatment failure. This was then followed by a brief overview of the pathophysiology of allergy in general, before moving into a more detailed discussion of this in the context of seasonal allergic rhinitis and asthma. Embedded within the programme were practical sessions on nasal spray and inhaler device technique. Delegates were given a copy of the British Society of Allergy & Clinical Immunology allergic rhinitis algorithm,19 and all treatment discussions were based on the World Health Organization’s Allergic Rhinitis and Asthma23 and British Thoracic Society/Scottish Intercollegiate Guidelines Network (http://www.sign.ac.uk/guidelines/fulltext/101/index.html) guidelines on the management of allergic rhinitis and asthma, respectively (current at the time of the study). There was ample time for individual and group discussions to ensure that individual learning styles were catered for and any queries were addressed.
Health-care professionals (1 general practitioner and 19 practice nurses) nominated by the general practices who were randomised to the intervention arm attended these 1-day intensive workshops delivered by experienced Education for Health trainers. The course was repeated on five occasions.
Control group
Health-care professionals (again nominated by the practices and in this case all nurses) in the control practices received an allergic rhinitis algorithm developed by the British Society of Allergy & Clinical Immunology,19 which was adapted for use in primary care by Education for Health. No training was offered to the 18 health-care professionals randomised to the control arm.
Objectives and outcomes
Our primary outcome of interest was the difference in the change in adolescents Standardised Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ(S)) score between baseline and 6 weeks post intervention in the intervention arm compared with the control arm. Consenting patients completed the RQLQ(S) and a symptoms score, which was measured on a 10-point visual analogue scale. Data were collected from two cohorts of patients in two separate years: 2009 and 2010. Baseline RQLQ(S) and symptom scores were recorded in May and early June 2009/2010; patients were then seen by their health-care professional, and follow-up RQLQ(S) and symptom scores were recorded in late June and July 2009/2010 (i.e., during the peak of the grass pollen season).
Our secondary outcomes of interest were:
-
Assessment of change in clinical practice among the health-care professionals allocated to the intervention arm: the effects of the 1-day training on self-reported professional confidence, and understanding and clinical management of seasonal allergic rhinitis, which were measured immediately before and after the training days, and after all the patients taking part in the study had been seen by a health-care professional (~7–28 days), using a questionnaire that incorporated a 5-point Likert scale (1=less confident and 5=more confident).
-
Total number of consultations for seasonal allergic rhinitis.
-
Total number of prescribed medications for seasonal allergic rhinitis.
-
Patient-reported symptom scores.
Pollen data
Grass pollen data from York and Edinburgh (which covered the areas from which practices were drawn) were centrally provided by the National Pollen and Aerobiology Research Unit for 2009 and 2010 in order to assess whether the pollen counts reached a level that was likely to trigger seasonal allergic rhinitis symptoms during the study period. The pollen count is a measure of the number of pollen grains per cubic metre (pg/m3) of air sampled, averaged over 24 h.
Sample size calculations
We calculated that a target sample size of 220 would give 80% power to detect a mean difference of 0·5 in RQLQ(S) score—the minimal clinically important difference at the 5% significance level. This was calculated using Sampsize24 assuming an s.d. of 1.225 and intraclass correlation (ICC) of 0.02. There is little evidence in the literature of the likely size of the design effect from clustering in trials of this kind, and the choice of the relatively low ICC value of 0.02 was based mainly on the finding of no evidence of clustering in our earlier adult study using RQLQ.14
Statistical analysis
We undertook a complete case analysis for our main analysis. In the primary analysis, multilevel modelling using a random effects model was used to take account of between- and within-cluster variation, adjusting for strata, individual covariates and year of study. Estimates and confidence intervals of the intervention effects are reported for the RQLQ(S) and symptom score.
Consultation and prescribing data were collected from the participating general practices for all patients from the date of consultation for the study to 31 August 2009/2010. Differences between the two groups were analysed using multilevel analysis in MLWin (version 2.20, 2010, http://www.bristol.ac.uk/cmm/software/mlwin/).
Differences in the mean scores of self-reported confidence and understanding of seasonal allergic rhinitis management in the intervention group were compared using t-statistics.
We first used mixed-model analysis of variance (ANOVA) using SPSS (version 14, 2005, SPSS, Chicago, IL, USA) to estimate the ICC for RQLQ(S), followed by a Bayesian approach using WinBUGS (version 1.4, 2009, http://www2.mrc-bsu.cam.ac.uk/bugs/winbugs/contents.shtml), taking a uniform prior over 0–1.26
Deviations from the trial protocol
Educational data
We were unable to collect educational data because of time constraints resulting from the delays in recruiting practices.
Follow-up RQLQ(S)
Follow-up primary outcome data were collected by post and the majority were collected 6 weeks post intervention as planned and described in the trial protocol;20 however, there were a small number of questionnaires collected between 6 and 8 weeks post intervention as a result of non-responders to the initial mailing and the need to issue reminders.
Missing data
Our complete case analysis provided unbiased estimates under the assumption that the missing data are ‘Missing Completely At Random’;27 however, analysis of the means estimated separately for each pattern of missingness suggested that this assumption may not hold. We therefore carried out further sensitivity analyses testing a variety of assumptions:
-
The direct likelihood method:28 the primary analysis of the effect of the intervention on RQLQ(S) was repeated, but with the baseline score modelled jointly with the outcome at 6 weeks instead of entering the model through the linear predictor. This model allowed for the inclusion of all 309 patients with data on at least one occasion and provided likelihood-based estimates that are valid under ‘Missing At Random’27 (under the assumption that the responses are multivariate normal).
-
Multiple imputation: Proc MI in SAS (SAS Institute, Cary, NC, USA) was used to generate multiple imputations separately for each treatment arm (pooling across centres) (m=100 imputations).
-
Alternative scenarios under ‘Missing Not At Random’27 for poor and good outcomes: first, under a poor outcome assumption, we imputed missing values in any particular cluster (at baseline or follow-up) using the largest observed score from that cluster (and time point). Second, under a good outcome assumption, we used the lowest observed scores from each cluster (and time point) to impute missing values.
Results
Baseline characteristics
Thirty-eight general practices (clusters) agreed to participate in the study, of which 20 were randomised to the intervention arm and 18 to the control arm. Of the patients assessed for eligibility from the general practice medical records, 1,565 satisfied our inclusion criteria and, of them, 341 agreed to participate (see Figure 1).
Participating (n=38) and non-participating practices (n=204) had comparable demographic characteristics. In Scottish sites, the mean and s.d. of list size for participating practices was 6,562 (3,363) and that for non-participating practices was 6,971 (3,302) (P=0.75); the mean deprivation quintiles were 2.69 (s.d. 0.67) and 2.60 (s.d. 0.70) (P=0.76), respectively. In English sites, the mean list size for participating practices was 8,707 (s.d. 4,048) and for non-participating practices 7,464 (s.d. 4,847) (P=0.19); the Index of Multiple Deprivation scores were 26.8 (s.d. 18.2) and 31.0 (s.d. 19.6) (P=0.27), respectively.
Clusters were comparable for baseline characteristics in terms of deprivation; however, the intervention practices had a larger mean list size (see Table 1). Participants were comparable at baseline in terms of age and sex profiles.
Primary outcome: impact on seasonal allergic rhinitis quality of life
A total of 246/341 patients (50·2% male, mean age 15 years) were included in the primary outcome analysis. The intervention failed to result in a clinically important improvement in RQLQ(S) (−0.15, 95% confidence interval (CI), −0.52 to +0.21) (adjusted for baseline RQLQ(S)), practice list size and region, year of study and deprivation.29
Secondary outcomes
Assessment of change in clinical practice
Health-care professionals’ self-assessment of their confidence and understanding of seasonal allergic rhinitis management markedly increased post intervention when compared with the baseline assessment (see Table 2). All scores improved from Time 1 (immediately before the training day) to both Time 2 (immediately after the training day) and Time 3 (after all patients had been seen as part of the study).
Consultation and prescribing data
Five of the 38 practices did not provide data on consultation and prescribing patterns (three control and two intervention arm practices). Table 3 summarises data revealing that the intervention arm practices tended to have more consultations and prescriptions in total, and also more consultations for other respiratory conditions, but that the figures for seasonal allergic rhinitis did not differ greatly between the two arms.
Grass pollen and symptom score data
Figure 2 indicates that the grass pollen reached sufficiently high counts (50–149 pg/m3) at both sites in both years to induce seasonal allergic rhinitis symptoms and that there was no significant regional variation between the two collection sites. Adjusted symptom scores in the intervention group were slightly lower than in the control group (−0.24, 95% CI, −1.03 to +0.54).
Estimates of ICC
The ICC was estimated as 0.034 after adjusting for baseline RQLQ and the intervention group, with a 95% credible interval of 0.0016–0.145.
Sensitivity analysis for impact on quality of life
By using the direct likelihood method28 to account for the missing data under a Missing At Random mechanism, the (adjusted) effect of the novel intervention on RQLQ(S) at 6 weeks was found to be 0.03 (95% CI, −0.33 to 0.39), supporting the finding that there is no beneficial effect of the intervention on RQLQ(S) score.
We obtained similar results with multiple imputation methods based on 100 imputed data sets. Using multiple imputations to account for the missing data, the intervention effect was 0.06 (95% CI, −0.30 to 0.42).
Finally, under the poor outcomes scenario, imputing missing data using large RQLQ(S) values, the estimated effect of the intervention was 0.21 (95% CI, −0.21 to 0.63). Under the good outcomes scenario, using low RQLQ(S) values to impute the missing data, the estimated intervention effect was 0.03 (95% CI, −0.39 to 0.44). The conclusions were thus unchanged: the intervention failed to have the desired effect.
Discussion
Main findings
This large general practice–based cluster randomised controlled trial has shown that a short intensive evidence-based allergy workshop for health-care professionals led to substantial and persistent improvements in their self-reported confidence and understanding of the management of seasonal allergic rhinitis, but this did not translate into changes in clinical practice in terms of frequency of consultations or prescribing habits; most importantly, this did not lead to clinically significant improvements in disease-specific quality of life or symptom score in adolescents with seasonal allergic rhinitis.
Strengths and limitations of this study
The main strength of this trial was the decision formally to evaluate this evidence-based educational intervention using an adequately powered cluster randomised controlled trial, which is most uncommon. We developed a complex intervention based on a previous effective educational intervention for health-care professionals and measured the effectiveness of this on a validated disease-specific quality of life measure; in keeping with the Medical Research Council’s complex intervention framework,16 we also measured a range of relevant process measures, which aimed to shed light on the mechanisms through which any changes were mediated and/or blocked. We have demonstrated the acceptability of the intervention and that it has an impact on professionals’ self-assessed confidence and knowledge of seasonal allergic rhinitis management, but that the intervention did not equip individuals with the ability to enact relevant structural changes in their practices to translate this into improvements in care processes.
We conducted a complete case analysis supplemented by sensitivity analyses to assess the likely impact of the missing data. Our sensitivity analyses strengthened our finding of no beneficial effect of the intervention on RQLQ(S). A limitation of this study may be that patients in the control arm of the cluster trial also consulted with a health-care professional, which it could be argued may not reflect routine primary care. It was necessary to design the study this way in order to understand the cause of any potential effectiveness of the educational intervention and to be able to distinguish this from any impact of simply being seen for seasonal allergic rhinitis. Control arm practices received an algorithm and information leaflet for the management of seasonal allergic rhinitis, both of which were adapted by Education for Health. One way of disentangling this issue would have been to include a third arm in which practices received no intervention; however, this was not possible within the constraints of this trial. An additional possible limitation of this study is that cluster sizes were not balanced. We used general practice list size in the minimisation scheme; we may, however, have reached a better balance in clusters if we had used number of adolescents, as this varied between clusters more than we had anticipated. The effect of this imbalance on the power of the study was small, and we still achieved the power required.
Interpretation of findings in relation to previously published work
Patients in this study had on average relatively mild impairment of quality of life measured by the RQLQ(S), which is in line with a similar study exploring the quality of life of perennial rhinitis sufferers.14 Patients were recruited from a primary care setting, using a clinician diagnosis of seasonal allergic rhinitis or a prescription for drugs used in nasal allergy in the last 2 years, rather than objective evidence of moderate or severe disease. As also observed in our earlier perennial rhinitis trial,14 the impact of this training intervention may have been more evident if we had restricted trial entry to those with more severe disease. This would, however, have reduced the generalisability of the intervention to everyday general practice.
We would have expected more consultations in the intervention group if the training had achieved a sustained change in clinical practice, but this was not the case. The training days were delivered by practising health-care professionals and based on current evidence-based guidelines for the management of seasonal allergic rhinitis developed by the British Society of Allergy & Clinical Immunology.19 The prescribing data included all repeat prescriptions; therefore, if the intervention practices followed the guidance given in the workshop, patients with persistent symptoms would be receiving an antihistamine, nasal steroid and/or topical ocular treatments, as appropriate.
Implications for future research, policy and practice
Future trials need to build on the findings of both this and our earlier trial,14 and find ways of equipping participants of such short courses with the skills necessary to bridge the gap between knowledge and day-to-day practice. A key consideration is not only to upskill primary care–based health-care professionals but also to develop their ability to effect organisational change. The increasing opportunities for blended learning—i.e., a combination of both face-to-face and virtual training—should also provide opportunities for periodic, convenient and accessible reinforcement of key messages. Developing such initiatives and then formally trialling their effectiveness is important to help ensure that the National Health Service and other health-care systems internationally invest their limited resources in evidence-based educational interventions of proven effectiveness.
Conclusions
In conclusion, this intensive seasonal allergic rhinitis training workshop for primary care health-care professionals was found acceptable and increased self-assessed confidence in attendees, but this did not translate into improvements in symptom control or quality of life of adolescents with seasonal allergic rhinitis.
References
Punekar Y, Sheikh A . Estabishing the incidence and prevalence of clinician-diagnosed allergic conditions in children and adolescents using routinely collected data from general practices. Clin Exp Allergy 2009; 39: 1209–1216.
Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733–743.
Bjorksten B, Clayton T, Ellwood P, Stewart A, Strachan D Group IPIS. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol 2008; 19: 110–124.
International Study of Asthma and Allergies in Childhood. Worldwide variations in the prevalence of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998; 351: 1225–1232.
Juniper EF, Guyatt GH, Dolovich J . Assessment of quality of life in adolescents with allergic rhinoconjunctivitis. Development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol 1994; 93: 413–423.
Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A . Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol 2007; 120: 381–387.
Blaiss MS Allergic Rhinitis in Schoolchildren Consensus Group. Allergic rhinitis and impairment issues in schoolchildren: a consensus report. Curr Med Res Opin 2004; 20: 1937–1952.
Greiner AN, Hellings PW, Rotiroti G, Scadding GK . Allergic rhinitis. Lancet 2011; 378: 2112–2122.
Lancet T . Allergic rhinitis: common, costly, and neglected. Lancet 2008; 371: 2057–2057.
Agency for Healthcare Research and QualityAllergic Rhinitis: Trends in Use and Expenditures, 2000 and 2005 2008.
Bauchau V, Durham SR . Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004; 24: 758–764.
Ryan D, Grant-Casey J, Scadding GK, Pereira S, Pinnock H, Sheikh A . Management of allergic rhinitis in UK primary care: baseline audit. Prim Care Respir J 2005; 14: 204–209.
Ghouri N, Hippisley-Cox J, Newton J, Sheikh A . Trends in the epidemiology and prescribing of medication for allergic rhinitis in England. J R Soc Med 2008; 101: 466–472.
Sheikh A, Khan-Wasti S, Price D, Smeeth L, Fletcher M, Walker S . Standardized training for healthcare professionals and its impact on patients with perennial rhinitis: a multi-centre randomised controlled trial. Clin Exp Allergy 2007; 37: 90–99.
Medical Research Council. A Framework for the Development and Evaluation of RCTs for Complex Interventions to Improve Health. MRC: London, UK, 2000.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337: a1655.
Hazeldine M, Worth A, Levy ML, Sheikh A . Follow-up survey of general practitioners' perceptions of UK allergy services. Prim Care Resp J 2010; 19: 84–86.
Levy ML, Price D, Zheng X, Simpson C, Hannaford P, Sheikh A . Inadequacies in UK primary care allergy services: national survey of current provisions and perceptions of need. Clin Exp Allergy 2004; 34: 518–519.
Scadding GK, Durham SR, Mirakian R, Jones NS, Leech S, Farooque CS et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy 2008; 38: 19–42.
Hammersley V, Elton R, Walker S, Sheikh A . Protocol for the adolescent hayfever trial: cluster randomised controlled trial of an educational intervention for healthcare professionals for the management of school-age children with hayfever. Trials 2010; 11: 84.
Hammersley V, Flint R, Pinnock H, Sheikh A . Developing and testing search strategies to identify patients with active seasonal allergic rhinitis in general practice. Prim Care Resp J 2011; 20: 71–74.
Carter B, Hood K . Balance algorithm for cluster randomised trials. BMC Med Res Methodol 2008; 8: 65.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy 2008; 63: 8–160.
Campbell MK, Thomson S, Ramsay CR, MacLennan GS, Grimshaw JM . Sample size calculator for cluster randomised trials. Comput Biol Med 2004; 34: 113–125.
Juniper EF, Thompson AK, Roberts JN . Can the standard gamble and rating scale be used to measure quality of life in rhinoconjunctivitis? Comparison with the RQLQ and SF-36. Allergy 2002; 57: 201–206.
Spiegelhalter D . Bayesian methods for cluster randomized trials with continuous responses. Stat Med 2001; 20: 435–452.
Rubin DB . Inference and missing data. Biometrika 1976; 72: 359–364.
Molenberghs G, Kenward MG (eds). Missing Data in Clinical Studies. Wiley: UK, 2007.
Juniper EF, Guyatt GH, Willan A, Griffith LE . Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994; 47: 81–87.
Acknowledgements
We thank the Scottish Primary Care Research Network and the Northern and Yorkshire Research Network for their help in recruiting general practices, the health-care professionals for attending the training and delivering the intervention, and the patients for volunteering to take part in the trial. We thank members of our Independent Trial Steering Committee for their oversight and support: Professor Tony Avery (Chair), Dr Sarah Armstrong, Dr Glenis Scadding and Dr Sarah Rodgers. The National Pollen and Aerobiology Research Unit at the University of Worcester, Dr Eric Caulton and Dr Katherine Selby kindly provided the pollen data.
Trial registration: Current Controlled Trials ISRCTN95538067
The views presented here are those of the author and not necessarily those of The Commonwealth Fund, its directors, officers, or staff.
Author information
Authors and Affiliations
Contributions
AS, SW and VSH conceived and managed the trial, AS is the guarantor. RAE provided statistical and methodological advice in designing the trial, CHH carried out post hoc sensitivity analysis. VSH was the researcher employed on this project and led the drafting of this manuscript. All authors commented on draft versions and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
SW is an Associate editor of, and AS is Joint Editor-in-Chief of, the PCRJ. Neither were involved in the editorial review of, nor the decision to publish, this article. All other authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hammersley, V., Elton, R., Walker, S. et al. Adolescent seasonal allergic rhinitis and the impact of health-care professional training: cluster randomised controlled trial of a complex intervention in primary care. npj Prim Care Resp Med 24, 14012 (2014). https://doi.org/10.1038/npjpcrm.2014.12
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/npjpcrm.2014.12