Abstract
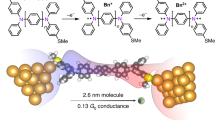
Fabricating nanoscopic devices capable of manipulating and processing single units of charge is an essential step towards creating functional devices where quantum effects dominate transport characteristics. The archetypal single-electron transistor comprises a small conducting or semiconducting island separated from two metallic reservoirs by insulating barriers1,2,3,4,5. By enabling the transfer of a well-defined number of charge carriers between the island and the reservoirs, such a device may enable discrete single-electron operations6,7,8,9. Here, we describe a single-molecule junction comprising a redox-active, atomically precise cobalt chalcogenide cluster wired between two nanoscopic electrodes10,11. We observe current blockade at room temperature in thousands of single-cluster junctions. Below a threshold voltage, charge transfer across the junction is suppressed. The device is turned on when the temporary occupation of the core states by a transiting carrier is energetically enabled, resulting in a sequential tunnelling process and an increase in current by a factor of ∼600. We perform in situ and ex situ cyclic voltammetry as well as density functional theory calculations to unveil a two-step process mediated by an orbital localized on the core of the cluster in which charge carriers reside before tunnelling to the collector reservoir. As the bias window of the junction is opened wide enough to include one of the cluster frontier orbitals, the current blockade is lifted and charge carriers can tunnel sequentially across the junction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Andres, R. P. et al. ‘Coulomb staircase’ at room temperature in a self-assembled molecular nanostructure. Science 272, 1323–1325 (1996).
Chen, S. et al. Gold nanoelectrodes of varied size: transition to molecule-like charging. Science 280, 2098–2101 (1998).
Klein, D. L., Roth, R., Lim, A. K. L., Alivisatos, A. P. & McEuen, P. L. A single-electron transistor made from a cadmium selenide nanocrystal. Nature 389, 699–701 (1997).
Liang, W. J., Shores, M. P., Bockrath, M., Long, J. R. & Park, H. Kondo resonance in a single-molecule transistor. Nature 417, 725–729 (2002).
Park, J. et al. Coulomb blockade and the Kondo effect in single-atom transistors. Nature 417, 722–725 (2002).
Albrecht, T., Guckian, A., Ulstrup, J. & Vos, J. G. Transistor-like behavior of transition metal complexes. Nano Lett. 5, 1451–1455 (2005).
Perrin, M. L., Burzurí, E. & van der Zant, H. S. Single-molecule transistors. Chem. Soc. Rev. 44, 902–919 (2015).
Su, T. A., Neupane, M., Steigerwald, M. L., Venkataraman, L. & Nuckolls, C. Chemical principles of single-molecule electronics. Nat. Rev. Mater. 1, 16002 (2016).
Kubatkin, S. et al. Single-electron transistor of a single organic molecule with access to several redox states. Nature 425, 698–701 (2003).
Choi, B. et al. Solvent-dependent conductance decay constants in single cluster junctions. Chem. Sci. 7, 2701–2705 (2016).
Roy, X. et al. Quantum soldering of individual quantum dots. Angew. Chem. Int. Ed. 51, 12473–12476 (2012).
Kano, S., Tada, T. & Majima, Y. Nanoparticle characterization based on STM and STS. Chem. Soc. Rev. 44, 970–987 (2015).
Xu, B. Q. & Tao, N. J. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science 301, 1221–1223 (2003).
Capozzi, B. et al. Single-molecule diodes with high rectification ratios through environmental control. Nat. Nanotech. 10, 522–527 (2015).
Nagahara, L. A., Thundat, T. & Lindsay, S. M. Preparation and characterization of STM tips for electrochemical studies. Rev. Sci. Instrum. 60, 3128–3130 (1989).
Capozzi, B. et al. Mapping the transmission functions of single-molecule junctions. Nano Lett. 16, 3949–3954 (2016).
Gonzalez, M. T. et al. Electrical conductance of molecular junctions by a robust statistical analysis. Nano Lett. 6, 2238–2242 (2006).
Kamenetska, M. et al. Formation and evolution of single-molecule junctions. Phys. Rev. Lett. 102, 126803 (2009).
Yanson, A. I., Bollinger, G. R., van den Brom, H. E., Agrait, N. & van Ruitenbeek, J. M. Formation and manipulation of a metallic wire of single gold atoms. Nature 395, 783–785 (1998).
Capozzi, B. et al. Tunable charge transport in single-molecule junctions via electrolytic gating. Nano Lett. 14, 1400–1404 (2014).
Osorio, H. M. et al. Electrochemical single-molecule transistors with optimized gate coupling. J. Am. Chem. Soc. 137, 14319–14328 (2015).
Kay, N. J. et al. Single-molecule electrochemical gating in ionic liquids. J. Am. Chem. Soc. 134, 16817–16826 (2012).
Kouwenhoven, L. P. et al. Electron transport in quantum dots. Mesoscopic Electron Transport 345, 105–214 (1997).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Theory and Applications (Wiley, 2001).
Schwarz, F. et al. Field-induced conductance switching by charge-state alternation in organometallic single-molecule junctions. Nat. Nanotech. 11, 170–176 (2015).
Li, Z. et al. Regulating a benzodifuran single molecule redox switch via electrochemical gating and optimization of molecule/electrode coupling. J. Am. Chem. Soc. 136, 8867–8870 (2014).
Wang, X. B. et al. Probing the intrinsic electronic structure of the cubane [4Fe–4S] cluster: nature's favorite cluster for electron transfer and storage. J. Am. Chem. Soc. 125, 14072–14081 (2003).
Degroot, M. W. & Corrigan, J. F. in Comprehensive Coordination Chemistry II (ed. Meyer, T. J.) 57–123 (Pergamon, 2003).
Cecconi, F., Ghilardi, C. A. & Midollini, S. Synthesis and structural characterization of a paramagnetic octahedral cobalt-sulfur cluster, [Co6µ3-S)8(PEt3)6] BPh4 . Inorg. Chim. Acta 64, L47–L48 (1982).
Widawsky, J. R. et al. Measurement of voltage-dependent electronic transport across amine-linked single-molecular-wire junctions. Nanotechnology 20, 434009 (2009).
Acknowledgements
This work was supported primarily by the Center for Precision Assembly of Superstratic and Superatomic Solids at Columbia University, an NSF MRSEC (award no. DMR-1420634). B.C. is supported by a NSF Graduate Research Fellowship under grant no. DGE 11-44155. X-ray diffraction measurements were performed in the Shared Materials Characterization Laboratory at Columbia University.
Author information
Authors and Affiliations
Contributions
Experiments were conceived and designed by G.L., B.C., M.L.S., X.R. and L.V. and performed by G.L. Compounds were synthesized and characterized by B.C. DFT calculations were performed by M.L.S. SCXRD of all compounds was performed by B.C. and D.W.P. The manuscript was co-written by G.L., B.C., M.L.S., X.R. and L.V., with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information 1 (PDF 1465 kb)
Supplementary information
Supplementary information 2 (XLSX 11 kb)
Rights and permissions
About this article
Cite this article
Lovat, G., Choi, B., Paley, D. et al. Room-temperature current blockade in atomically defined single-cluster junctions. Nature Nanotech 12, 1050–1054 (2017). https://doi.org/10.1038/nnano.2017.156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2017.156
This article is cited by
-
Single-cluster electronics using metallic clusters: Fabrications, regulations, and applications
Nano Research (2024)
-
Voltage-driven control of single-molecule keto-enol equilibrium in a two-terminal junction system
Nature Communications (2023)
-
Non-covalent interaction-based molecular electronics with graphene electrodes
Nano Research (2023)
-
Dual-gated single-molecule field-effect transistors beyond Moore’s law
Nature Communications (2022)
-
σ–σ Stacked supramolecular junctions
Nature Chemistry (2022)