Abstract
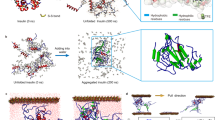
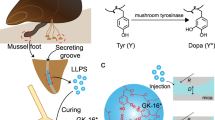
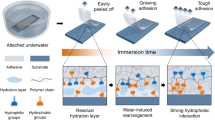
Many natural underwater adhesives harness hierarchically assembled amyloid nanostructures to achieve strong and robust interfacial adhesion under dynamic and turbulent environments. Despite recent advances, our understanding of the molecular design, self-assembly and structure–function relationships of these natural amyloid fibres remains limited. Thus, designing biomimetic amyloid-based adhesives remains challenging. Here, we report strong and multi-functional underwater adhesives obtained from fusing mussel foot proteins (Mfps) of Mytilus galloprovincialis with CsgA proteins, the major subunit of Escherichia coli amyloid curli fibres. These hybrid molecular materials hierarchically self-assemble into higher-order structures, in which, according to molecular dynamics simulations, disordered adhesive Mfp domains are exposed on the exterior of amyloid cores formed by CsgA. Our fibres have an underwater adhesion energy approaching 20.9 mJ m−2, which is 1.5 times greater than the maximum of bio-inspired and bio-derived protein-based underwater adhesives reported thus far. Moreover, they outperform Mfps or curli fibres taken on their own and exhibit better tolerance to auto-oxidation than Mfps at pH ≥ 7.0.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dolgin, E. The sticking point. Nature Med. 19, 124–125 (2013).
Lee, B. P., Messersmith, P. B., Israelachvili, J. N. & Waite, J. H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res., 41, 99–132 (2011).
Brubaker, C. E. & Messersmith, P. B. The present and future of biologically inspired adhesive interfaces and materials. Langmuir 28, 2200–2205 (2012).
Stewart, R. J., Ransom, T. C. & Hlady, V. Natural underwater adhesives. J. Polym. Sci. B 49, 757–771 (2011).
Stewart, R. J. Protein-based underwater adhesives and the prospects for their biotechnological production. Appl. Microbiol. Biotechnol. 89, 27–33 (2011).
Yin, M., Yuan, Y., Liu, C. S. & Wang, J. Development of mussel adhesive polypeptide mimics coating for in-situ inducing re-endothelialization of intravascular stent devices. Biomaterials 30, 2764–2773 (2009).
Brubaker, C. E., Kissler, H., Wang, L. J., Kaufman, D. B. & Messersmith, P. B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials 31, 420–427 (2010).
Matos-Perez, C. R., White, J. D. & Wilker, J. J. Polymer composition and substrate influences on the adhesive bonding of a biomimetic, cross-linking polymer. J. Am. Chem. Soc. 134, 9498–9505 (2012).
Hwang, D. S., Yoo, H. J., Jun, J. H., Moon, W. K. & Cha, H. J. Expression of functional recombinant mussel adhesive protein Mgfp-5 in Escherichia coli. Appl. Environ. Microbiol. 70, 3352–3359 (2004).
Kamino, K., Nakano, M. & Kanai, S. Significance of the conformation of building blocks in curing of barnacle underwater adhesive. FEBS J. 279, 1750–1760 (2012).
Kamino, K. Underwater adhesive of marine organisms as the vital link between biological science and material science. Marine Biotechnol. 10, 111–121 (2008).
Anika, S. in The Functional Fold: Amyloid Structures in Nature (ed. Mostaert, S. J. A.) 131–146 (Pan Stanford, 2012).
Barlow, D. E. et al. Characterization of the adhesive plaque of the barnacle Balanus amphitrite: amyloid-like nanofibrils are a major component. Langmuir 26, 6549–6556 (2010).
Wasmer, C. et al. Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science 319, 1523–1526 (2008).
Sawaya, M. R. et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447, 453–457 (2007).
Knowles, T. P. J. & Buehler, M. J. Nanomechanics of functional and pathological amyloid materials. Nature Nanotech. 6, 469–479 (2011).
Knowles, T. P. et al. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 318, 1900–1903 (2007).
Chapman, M. R. et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855 (2002).
Knowles, T. P. J., Oppenheim, T. W., Buell, A. K., Chirgadze, D. Y. & Welland, M. E. Nanostructured films from hierarchical self-assembly of amyloidogenic proteins. Nature Nanotech. 5, 204–207 (2010).
Li, C. X., Adamcik, J. & Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nature Nanotech. 7, 421–427 (2012).
Waite, J. H. & Benedict, C. V. Assay of dihydroxyphenylalanine (dopa) in invertebrate structural proteins. Methods Enzymol. 107, 397–413 (1983).
Paz, M., Flückiger, R., Boak, A., Kagan, H. & Gallop, P. M. Specific detection of quinoproteins by redox-cycling staining. J. Biol. Chem. 266, 689–692 (1991).
Wang, X., Zhou, Y., Ren, J-J., Hammer, N. D. & Chapman, M. R. Gatekeeper residues in the major curlin subunit modulate bacterial amyloid fiber biogenesis. Proc. Natl Acad. Sci. USA 107, 163–168 (2010).
Sugase, K., Dyson, H. J. & Wright, P. E. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447, 1021–1025 (2007).
Yang, J. et al. Development of aliphatic biodegradable photoluminescent polymers. Proc. Natl Acad. Sci. USA 106, 10086–10091 (2009).
Williams, A. T. R., Winfield, S. A. & Miller, J. N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 108, 1067–1071 (1983).
Del Mercato, L. L. et al. Charge transport and intrinsic fluorescence in amyloid-like fibrils. Proc. Natl Acad. Sci. USA 104, 18019–18024 (2007).
Smith, G. J. The fluorescence of dihydroxyphenylalanine: the effects of protonation–deprotonation. Color. Technol. 115, 346–349 (1999).
Chen, R. F. Fluorescence quantum yields of tryptophan and tyrosine. Anal. Lett. 1, 35–42 (1967).
Al-Hilaly, Y. K. et al. A central role for dityrosine crosslinking of amyloid-β in Alzheimer's disease. Acta Neuropathol. Commun. 1, 83 (2013).
Leite, F. & Herrmann, P. Application of atomic force spectroscopy (AFS) to studies of adhesion phenomena: a review. J. Adhes. Sci. Technol. 19, 365–405 (2005).
Danner, E. W., Kan, Y. J., Hammer, M. U., Israelachvili, J. N. & Waite, J. H. Adhesion of mussel foot protein Mefp-5 to mica: an underwater superglue. Biochemistry 51, 6511–6518 (2012).
Wei, W., Yu, J., Broomell, C., Israelachvili, J. N. & Waite, J. H. Hydrophobic enhancement of dopa-mediated adhesion in a mussel foot protein. J. Am. Chem. Soc. 135, 377–383 (2012).
Wu, C., Lim, J. Y., Fuller, G. G. & Cegelski, L. Quantitative analysis of amyloid-integrated biofilms formed by uropathogenic Escherichia coli at the air–liquid interface. Biophys. J. 103, 464–471 (2012).
Goulter-Thorsen, R., Taran, E., Gentle, I., Gobius, K. & Dykes, G. CsgA production by Escherichia coli O157: H7 alters attachment to abiotic surfaces in some growth environments. Appl. Environ. Microbiol. 77, 7339–7344 (2011).
Lu, Q. et al. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 10, 20120759 (2013).
Yu, J. et al. Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films. Proc. Natl Acad. Sci. USA 110, 15680–15685 (2013).
Li, Y., Qin, M., Li, Y., Cao, Y. & Wang, W. Single molecule evidences for the adaptive binding of DOPA to different wet surfaces. Langmuir 30, 4358–4366 (2014).
Fowler, D. M., Koulov, A. V., Balch, W. E. & Kelly, J. W. Functional amyloid—from bacteria to humans. Trends Biochem. Sci. 32, 217–224 (2007).
Weber, W. & Fussenegger, M. Emerging biomedical applications of synthetic biology. Nature Rev. Genet. 13, 21–35 (2012).
Chen, A. Y. et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nature Mater. 13, 515–523 (2014).
Sinclair, J. C., Davies, K. M., Venien-Bryan, C. & Noble, M. E. M. Generation of protein lattices by fusing proteins with matching rotational symmetry. Nature Nanotech. 6, 558–562 (2011).
Lv, S. et al. Designed biomaterials to mimic the mechanical properties of muscles. Nature 465, 69–73 (2010).
Qian, L. & Winfree, E. Scaling up digital circuit computation with DNA strand displacement cascades. Science 332, 1196–1201 (2011).
Auslander, S., Auslander, D., Muller, M., Wieland, M. & Fussenegger, M. Programmable single-cell mammalian biocomputers. Nature 487, 123–127 (2012).
Hong, S. H. et al. Cell-free protein synthesis from a release factor 1 deficient Escherichia coli activates efficient and multiple site-specific non-standard amino acid incorporation. ACS Synth. Biol. 3, 398–409 (2014).
Lajoie, M. J. et al. Genomically recoded organisms expand biological functions. Science 342, 357–360 (2013).
Eswar, N. et al. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinf. 5.6.1–5.6.30 (2006).
Hwang, D. S. & Waite, J. H. Three intrinsically unstructured mussel adhesive proteins, mfp-1, mfp-2, and mfp-3: analysis by circular dichroism. Protein Sci. 21, 1689–1695 (2012).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy (Springer, 2009).
Acknowledgements
The authors thank A. Schwartzman for help in applying AFM colloid nanoparticle technology for measuring adhesive forces and H. Tavakoli Nia (Ortiz group, MIT) for initial discussions regarding this technology. The authors acknowledge help from the NERCE Biomolecule Production Laboratory (Harvard University) for producing part of the cell pellets for protein purification. The authors also thank the Whitehead Institute and the Biopolymers Laboratory in the David H. Koch Institute for Integrated Cancer Research and the Institute for Soldier Nanotechnologies for access to characterization equipment. This research was primarily supported by the Office of Naval Research (N000141310647). This work was also supported in part by the MRSEC Program of the National Science Foundation under award no. DMR-0819762. T.K.L. acknowledges support from the NIH New Innovator Award (1DP2OD008435). The molecular dynamics modelling used the computer cluster and corresponding materials based upon work supported by the National Science Foundation under award no. 0821391.
Author information
Authors and Affiliations
Contributions
T.K.L. directed the research. C.Z. conceived the technical details and designed the experiments. C.Z. performed or participated in all the experiments. J.D. performed experiments in protein expression and purification. Z.D. assisted in collecting and analysing the fluorescence emission and excitation spectra. A.C. constructed the genes. C.M.S. and T.G. designed the simulations. T.G. performed the simulations. C.Z. and T.K.L. wrote the manuscript with help from all authors. All authors contributed to revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.K.L. and C.Z. have filed a patent disclosure with the MIT Technology Licensing Office on this work.
Supplementary information
Supplementary information
Supplementary Information (PDF 3808 kb)
Rights and permissions
About this article
Cite this article
Zhong, C., Gurry, T., Cheng, A. et al. Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nature Nanotech 9, 858–866 (2014). https://doi.org/10.1038/nnano.2014.199
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2014.199
This article is cited by
-
Programmable adhesion and morphing of protein hydrogels for underwater robots
Nature Communications (2024)
-
Applications of synthetic biology in medical and pharmaceutical fields
Signal Transduction and Targeted Therapy (2023)
-
The mussel Mytilus galloprovincialis (Crimea, Black Sea) as a source of essential trace elements in human nutrition
Biological Trace Element Research (2023)
-
Engineering Mechanical Strong Biomaterials Inspired by Structural Building Blocks in Nature
Chemical Research in Chinese Universities (2023)
-
In silico prediction and in vitro validation of the effect of pH on adhesive behaviour of the fused CsgA-MFP3 protein
AMB Express (2022)