Abstract
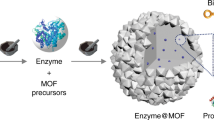
Flower-shaped inorganic nanocrystals1,2,3 have been used for applications in catalysis4,5 and analytical science6,7, but so far there have been no reports of ‘nanoflowers’ made of organic components8. Here, we report a method for creating hybrid organic–inorganic nanoflowers using copper (II) ions as the inorganic component and various proteins as the organic component. The protein molecules form complexes with the copper ions, and these complexes become nucleation sites for primary crystals of copper phosphate. Interaction between the protein and copper ions then leads to the growth of micrometre-sized particles that have nanoscale features and that are shaped like flower petals. When an enzyme is used as the protein component of the hybrid nanoflower, it exhibits enhanced enzymatic activity and stability compared with the free enzyme. This is attributed to the high surface area and confinement of the enzymes in the nanoflowers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Song, Y. et al. Controlled synthesis of 2-D and 3-D dendritic platinum nanostructures. J. Am. Chem. Soc. 126, 635–645 (2004).
Narayanaswamy, A., Xu, H., Pradhan, N., Kim, M. & Peng, X. Formation of nearly monodisperse In2O3 nanodots and oriented-attached nanoflowers: hydrolysis and alcoholysis vs pyrolysis. J. Am. Chem. Soc. 128, 10310–10319 (2006).
Sun, Z. et al. Rational design of 3D dendritic TiO2 nanostructures with favorable architectures. J. Am. Chem. Soc. 133, 19314–19317 (2011).
Lim, B. et al. Pd–Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 324, 1302–1305 (2009).
Mohanty, A., Garg, N. & Jin, R. A universal approach to the synthesis of noble metal nanodendrites and their catalytic properties. Angew. Chem. Int. Ed. 49, 4962–4966 (2010).
Xie, J., Zhang, Q., Lee, J. Y. & Wang, D. I. C. The synthesis of SERS-active gold nanoflower tags for in vivo applications. ACS Nano 2, 2473–2480 (2008).
Jia, W., Su, L. & Lei, Y. Pt nanoflower/polyaniline composite nanofibers based urea biosensor. Biosens. Bioelectron. 30, 158–164 (2011).
Kharisov, B. I. A review for synthesis of nanoflowers. Recent Pat. Nanotechnol. 2, 190–200 (2008).
Harford, C. & Sarkar, B. Amino terminal Cu(II) and Ni(II)-binding (ATCUN) motif of proteins and peptides. Acc. Chem. Res. 30, 123–130 (1997).
Smith, P. K. et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 (1985).
Rulíšek, L. & Vondrášek, J. Coordination geometries of selected transition metal ions (Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Hg2+) in metalloproteins. J. Inorg. Biochem. 71, 115–127 (1998).
Chandra, N., Brew, K. & Acharya, K. R. Structural evidence for the presence of a secondary calcium binding site in human alpha-lactalbumin. Biochemistry 37, 4767–4772 (1998).
Piontek, K., Antorini, M. & Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J. Biol. Chem. 277, 37663–37669 (2002).
Saito, R., Sato, T., Ikai, A. & Tanaka, N. Structure of bovine carbonic anhydrase II at 1.95 Å resolution. Acta Crystallogr. D 60, 792–795 (2004).
Ericsson, D. J. et al. X-ray structure of Candida antarctica lipase A shows a novel lid structure and a likely mode of interfacial activation. J. Mol. Biol. 376, 109–119 (2008).
Kudva, Y. C., Sawka, A. M. & Young, W. F. Jr Clinical review 164: the laboratory diagnosis of adrenal pheochromocytoma: the Mayo Clinic experience. J. Clin. Endocrinol. Metab. 88, 4533–4539 (2003).
Morita, E. & Nakamura, E. Solid-phase extraction of antipyrine dye for spectrophotometric determination of phenolic compounds in water. Anal. Sci. 27, 489–492 (2011).
Kim, J., Grate, J. W. & Wang, P. Nanobiocatalysis and its potential applications. Trends Biotechnol. 26, 639–646 (2008).
Ge, J., Lu, D., Liu, Z. X. & Liu, Z. Recent advances in nanostructured biocatalysts. Biochem. Eng. J. 44, 53–59 (2009).
Luckarift, H. R., Spain, J. C., Naik, R. R. & Stone, M. O. Enzyme immobilization in a biomimetic silica support. Nature Biotechnol. 22, 211–213 (2004).
Mateo, C. et al. Immobilization of enzymes on heterofunctional epoxy supports. Nature Protoc. 2, 1022–1027 (2007).
Kim, J. & Grate, J. W. Single-enzyme nanoparticles armored by a nanometer-scale organic/inorganic network. Nano Lett. 3, 1219–1222 (2003).
Yan, M., Ge, J., Liu, Z. & Ouyang, P. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J. Am. Chem. Soc. 128, 11008–11009 (2006).
Ge, J. et al. Molecular fundamentals of enzyme nanogels. J. Phys. Chem. B 112, 14319–14324 (2008).
Ge, J., Lu, D., Wang, J. & Liu, Z. Lipase nanogel catalyzed transesterification in anhydrous dimethyl sulfoxide. Biomacromolecules 10, 1612–1618 (2009).
Yan, M., Liu, Z. X., Lu, D. & Liu, Z. Fabrication of single carbonic anhydrase nanogel against denaturation and aggregation at high temperature. Biomacromolecules 8, 560–565 (2007).
Lei, C., Shin, Y., Liu, J. & Ackerman, E. J. Entrapping enzyme in a functionalized nanoporous support. J. Am. Chem. Soc. 124, 11242–11243 (2002).
Dulay, M. T., Baca, Q. J. & Zare, R. N. Enhanced proteolytic activity of covalently bound enzymes in photopolymerized sol gel. Anal. Chem. 77, 4604–4610 (2005).
Murugesan, K., Kim, Y-M., Jeon, J-R. & Chang, Y-S. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J. Hazard. Mater. 168, 523–529 (2009).
Acknowledgements
The authors thank J. Brauman and K. Holmberg for helpful discussions and R-L. Jia for helping with acquiring TEM images. All experimental work was performed at Stanford University and was financially supported by the US National Science Foundation (CBET-0827806).
Author information
Authors and Affiliations
Contributions
J.G., J.L. and R.N.Z. conceived and designed the experiments. J.G. and J.L. performed the experiments. J.G. and R.N.Z. analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3213 kb)
Rights and permissions
About this article
Cite this article
Ge, J., Lei, J. & Zare, R. Protein–inorganic hybrid nanoflowers. Nature Nanotech 7, 428–432 (2012). https://doi.org/10.1038/nnano.2012.80
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2012.80
This article is cited by
-
Catalyzing Transformation: Organo-Inorganic Materials Based Immobilized Lipases in the Ongoing Quest for Sustainable Biodiesel Production
Topics in Catalysis (2024)
-
Rational design of EDTA-incorporated nanoflowers as novel and effective endodontic disinfection against biofilms
Odontology (2024)
-
Plant-based zinc nanoflowers assisted molecularly imprinted polymer for the design of an electrochemical sensor for selective determination of abrocitinib
Microchimica Acta (2024)
-
Immobilization of Thermoplasma acidophilum Glucose Dehydrogenase and Isocitrate Dehydrogenase Through Enzyme-Inorganic Hybrid Nanocrystal Formation
Current Microbiology (2024)
-
Catalytic and Antioxidant Activity of Desmarestia menziesii Algae Extract Based Organic@İnorganic Hybrid Nanoflowers
Journal of Inorganic and Organometallic Polymers and Materials (2023)