Abstract
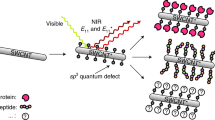
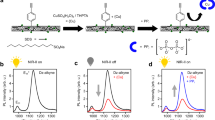
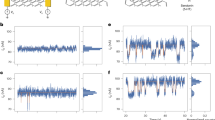
Biosensing applications of single-walled carbon nanotubes have been demonstrated in solid-state device structures1,2,3. Bioanalyte sensing schemes based on coupling of reversible nanotube fluorescence quenching to redox reactions paired to enzymatic peroxide generation have also been pursued4,5. Here we show a new approach to highly sensitive nanotube-based optical sensing. Single-walled carbon nanotubes interacting with dye–ligand conjugates—a redox-active dye molecule that is covalently bound to a biological receptor ligand (such as biotin in this case)—showed fluorescence quenching. Further interaction between the receptor ligand on the conjugates and target analytes (avidin in this case) induced the recovery of the quenched fluorescence, forming the basis of the sensing scheme. Nanomolar sensitivity was attained with high specificity for the target analyte. This is a versatile approach because a wide range of conjugation possibilities exists between the potential receptors and redox quenchers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Besteman, K., Lee, J.-O., Wiertz, F. G. M., Heering, H. A. & Dekker, C. Enzyme-coated carbon nanotubes as single-molecule biosensors. Nano Lett. 3, 727–730 (2003).
Chen, R. J. et al. Noncovalent functionalisation of carbon nanotubes for highly specific electronic biosensors. Proc. Natl Acad. Sci. USA 100, 4984–4989 (2003).
Katz, E. & Willner, I. Biomolecule-functionalized carbon nanotubes: applications in nanobioelectronics. ChemPhysChem. 5, 1084–1104 (2004).
Barone, P. W., Baik, S., Heller, D. A. & Strano, M. S. Near-infrared optical sensors based on single-walled carbon nanotubes. Nature Mater. 4, 86–92 (2005).
Song C., Pehrsson, P. E. & Zhao, W. Recoverable solution reaction of hipco carbon nanotubes with hydrogen peroxide. J. Phys. Chem. B. 109, 21634–21639 (2005).
O'Connell, M. J., Eibergen, E. E. & Doorn, S. K. Chiral selectivity in the charge-transfer bleaching of single-walled carbon-nanotube spectra. Nature Mater. 4, 412–418 (2005).
Chen, L. et al. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proc. Natl Acad. Sci. USA 96, 12287–12292 (1999).
Song, X., Wang, H.-L., Shi, J., Park, J. W. & Swanson, B. I. Conjugated polymers as efficient fluorescence quenchers and their applications for bioassays. Chem. Mater. 14, 2342–2347 (2002).
Dwight, S. J., Gaylord, B. S., Hong J. W. & Bazan, G. C. Perturbation of fluorescence by nonspecific interactions between anionic poly(phenylenevinylene)s and proteins: implications for biosensors. J. Am. Chem. Soc. 126, 16850–16859 (2004).
Wada, A. et al. Design and construction of glutamine binding proteins with a self-adhering capability to unmodified hydrophobic surfaces as reagentless fluorescence sensing devices. J. Am. Chem. Soc. 125, 16228–16234 (2003).
Albertorio, F. et al. Fluid and air-stable lipopolymer membranes for biosensor applications. Langmuir 21, 7476–7482 (2005).
Gruber, H. J. et al. Anomalous fluorescence enhancement of Cy3 and Cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalent linking to IgG and noncovalent binding to avidin. Bioconjugate Chem. 11, 696–704 (2000).
Dattelbaum, J. D. et al. Analysis of allosteric signal transduction mechanisms in an engineered fluorescent maltose biosensor. Protein Sci. 14, 284–291 (2005).
Hamblin, M. R., Miller, J. L. & Ortel, B. Scavenger-receptor targeted photodynamic therapy. Photochem. Photobio. 72, 533–540 (2000).
Feigenbaum, J. J., Choubal, M. D., Crumrine, D. S., Kanofsky, J. R. & Payza, K. Receptor inactivation by dye–neuropeptide conjugates. 3. Comparative binding of dye–neuropeptide conjugates to FMRFamide receptors of Helix aspersa and Loligo pealei. Peptides 17, 1279–1284 (1996).
Zheng, M. & Diner, B. A. Solution redox chemistry of carbon nanotubes. J. Am. Chem. Soc. 126, 15490–15494 (2004).
Dwight, S. J., Gaylord, B. S., Hong, J. W. & Bazan, G. C. Perturbation of fluorescence by nonspecific interactions between anionic poly(phenylenevinylene)s and proteins: Implications for biosensors. J. Am. Chem. Soc. 126, 16850–16859 (2004).
Haes, A. J. & Van Duyne, R. P. A highly sensitive and selective surface-enhanced nanobiosensor. Mater. Res. Soc. Symp. Proc. 723, 133–138, (2002).
Vikholm, I. & Albers, W. M. Oriented immobilization of antibodies for immunosensing. Langmuir 14, 3865–3872 (1998).
Acknowledgements
This work was carried out under the auspices of the National Nuclear Security Administration for the US Department of Energy at Los Alamos National Laboratory (LANL). Partial support was provided by the LANL Laboratory Directed Research and Development program.
Author information
Authors and Affiliations
Contributions
S.B.C., H.-L.W. and S.K.D. conceived and designed the experiments performed by S.B.C., L.O.B. and Y.G. designed and performed the DLC synthesis. C.-C.W. performed DLC synthesis and electrochemical measurements. All authors took part in co-writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure S1 and S2 (PDF 198 kb)
Rights and permissions
About this article
Cite this article
Satishkumar, B., Brown, L., Gao, Y. et al. Reversible fluorescence quenching in carbon nanotubes for biomolecular sensing. Nature Nanotech 2, 560–564 (2007). https://doi.org/10.1038/nnano.2007.261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2007.261
This article is cited by
-
Wearable chemical sensors for biomarker discovery in the omics era
Nature Reviews Chemistry (2022)
-
High-Precision Nonenzymatic Electrochemical Glucose Sensing Based on CNTs/CuO Nanocomposite
Journal of Electronic Materials (2022)
-
Analysis on the effect of ZnO on Carbon nanotube by spray pyrolysis method
Mechanics of Advanced Materials and Modern Processes (2016)
-
Emerging carbon-based nanosensor devices: structures, functions and applications
Advances in Manufacturing (2015)
-
Metal-particle-induced enhancement of the photoluminescence from biomolecule-functionalized carbon nanotubes
Nanoscale Research Letters (2014)