Abstract
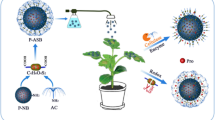
Surface-functionalized silica nanoparticles can deliver DNA1,2,3,4,5,6,7,8 and drugs9,10,11,12,13,14,15 into animal cells and tissues. However, their use in plants is limited by the cell wall present in plant cells. Here we show a honeycomb mesoporous silica nanoparticle (MSN) system with 3-nm pores that can transport DNA and chemicals into isolated plant cells and intact leaves. We loaded the MSN with the gene and its chemical inducer and capped the ends with gold nanoparticles to keep the molecules from leaching out. Uncapping the gold nanoparticles released the chemicals and triggered gene expression in the plants under controlled-release conditions. Further developments such as pore enlargement and multifunctionalization of these MSNs may offer new possibilities in target-specific delivery of proteins, nucleotides and chemicals in plant biotechnology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bharali, D. J. et al. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl Acad. Sci. USA 102, 11539–11544 (2005).
He, X. et al. A novel DNA-enrichment technology based on amino-modified functionalized silica nanoparticles. J. Disper. Sci. Technol. 24, 633–640 (2003).
Kneuer, C. et al. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro. Bioconjugate Chem. 11, 926–932 (2000).
Luo, D., Han, E., Belcheva, N. & Saltzman, W. M. A self-assembled, modular DNA delivery system mediated by silica nanoparticles. J. Control Release 95, 333–341 (2004).
Luo, D. & Saltzman, W. M. Enhancement of transfection by physical concentration of DNA at the cell surface. Nature Biotechnol. 18, 893–895 (2000).
Radu, D. R. et al. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 126, 13216–13217 (2004).
Roy, I. et al. Optical tracking of organically modified silica nanoparticles as DNA carriers: A nonviral, nanomedicine approach for gene delivery. Proc. Natl Acad. Sci. USA 102, 279–284 (2005).
Sameti, M. et al. Stabilisation by freeze-drying of cationically modified silica nanoparticles for gene delivery. Int. J. Pharm. 266, 51–60 (2003).
Giri, S., Trewyn, B. G., Stellmaker, M. P. & Lin, V. S.-Y. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. Int. Edn Engl. 44, 5038–5044 (2005).
Gruenhagen, J. A., Lai, C. Y., Radu, D. R., Lin, V. S.-Y. & Yeung, E. S. Real-time imaging of tunable adenosine 5-triphosphate release from an MCM-41-type mesoporous silica nanosphere-based delivery system. Appl. Spectrosc. 59, 424–431 (2005).
Hirsch, L. R. et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl Acad. Sci. USA 100, 13549–13554 (2003).
Huo, Q. et al. A new class of silica cross-linked micellar core–shell nanoparticles. J. Am. Chem. Soc. 128, 6447–6453 (2006).
Kulak, A., Hall, S. R. & Mann, S. Single-step fabrication of drug-encapsulated inorganic microspheres with complex form by sonication-induced nanoparticle assembly. Chem. Commun. 576–577 (2004).
Lai, C. Y. et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 125, 4451–4459 (2003).
Zhao, W., Gu, J., Zhang, L., Chen, H. & Shi, J. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J. Am. Chem. Soc. 127, 8916–8917 (2005).
Potrykus, I. Gene transfer to cereals: An assessment. Bio/Technology 8, 535–542 (1990).
Sheen, J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 127, 1466–1475 (2001).
Šamaj, J. Methods and molecular tools for studying endocytosis in plants: An overview in Plant Endocytosis (eds Šamaj, J., Baluška, F. & Menzel, D ), 1–17 (Springer, Berlin/Heidelberg, 2006).
Sheen, J. A transient expression assay using Arabidopsis mesophyll protoplasts. On www at http://genetics.mgh.harvard.edu/sheenweb/ (2002).
Shukla, R. et al. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 21, 10644–10654 (2005).
Huang, D.-M. et al. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: Implication for stem cell tracking. FASEB J. 19, 2014–2016 (2005).
Slowing, I., Trewyn, B. G. & Lin, V. S.-Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 128, 14792–14793 (2006).
Zuo, J., Niu, Q. W. & Chua, N. H. Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265–273 (2000).
Olhoft, P. M., Lin, K., Galbraith, J., Nielsen, N. C. & Somers, D. A. The role of thiol compounds in increasing Agrobacterium-mediated transformation of soybean cotyledonary-node cells. Plant Cell. Rep. 20, 731–737 (2001).
Zheng, M., Davidson, F. & Huang, X. Ethylene glycol monolayer protected nanoparticles for eliminating nonspecific binding with biological molecules. J. Am. Chem. Soc. 125, 7790–7791 (2003).
Brust, M., Fink, J., Bethell, D., Schiffrin, D. J. & Kiely, C. Synthesis and reactions of functionalized gold nanoparticles. J. Chem. Soc. Chem. Commun. 1655–1656 (1995).
Spangenberg, G. & Potrykus, I. (eds) Polyethylene glycol-mediated direct gene transfer to tobacco protoplasts in Gene Transfer to Plants 58–65 (Springer, Berlin/Heidelberg, 1995).
Frame, B. R. et al. Production of transgenic maize from bombarded type II callus: Effect of gold particle size and callus morphology on transformation efficiency. In Vitro Cell. Dev.-Pl. 36, 21–29 (2000).
Sanford, J. C., Smith, F. D. & Russell, J. A. Optimizing the biolistic process for different biological applications. Methods Enzymol. 217, 483–509 (1993).
Acknowledgements
We thank N.-H. Chua for providing pER8-GFP. Special thanks go to M. Carter for confocal microscope assistance, B. Frame and L. Moeller for discussions, and the Plant Transformation Facility personnel for providing maize immature embryos and maize callus media. The authors thank the Plant Science Institute at Iowa State University for financial support. V.S.-Y.L. thanks the U.S. NSF (CHE-0239570), the US DOE and the Office of Basic Energy Sciences (W-7405-Eng-82) for financial support for the synthesis and characterization of the MSN materials.
Correspondence regarding nanoparticles and requests for nanoparticle materials should be addressed to V.S.-Y.L.
Author information
Authors and Affiliations
Contributions
F.T. and K.W. conceived and designed the plant transformation experiments. F.T. performed the plant transformation experiments. V.S.-Y.L. and B.G.T. conceived and designed the surface functionalized mesoporous silica nanoparticle systems for the controlled release of DNAs and chemicals. B.G.T. performed experiments on the synthesis and characterization of the capped mesoporous silica nanoparticle materials. All authors discussed the results and participated in the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary methods and figures (PDF 1031 kb)
Rights and permissions
About this article
Cite this article
Torney, F., Trewyn, B., Lin, VY. et al. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nature Nanotech 2, 295–300 (2007). https://doi.org/10.1038/nnano.2007.108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2007.108
This article is cited by
-
Phytogenic nanoparticles: synthesis, characterization, and their roles in physiology and biochemistry of plants
BioMetals (2024)
-
The emerging role of nanotechnology in plant genetic engineering
Nature Reviews Bioengineering (2023)
-
Quantitative biodistribution of nanoparticles in plants with lanthanide complexes
Scientific Reports (2023)
-
Boosting wheat yield, profitability and NUE with prilled and nano urea in conservation tillage
Scientific Reports (2023)
-
Promoting genotype-independent plant transformation by manipulating developmental regulatory genes and/or using nanoparticles
Plant Cell Reports (2023)