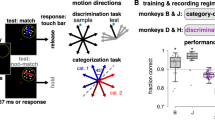
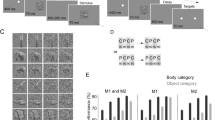
Abstract
Here we investigated the impact of visual discrimination training on neuronal responses to parts of images and to whole images in inferotemporal (IT) cortex. Monkeys were trained to discriminate among 'baton' stimuli consisting of discrete top and bottom parts joined by a vertical stem. With separate features at each end, we were able to manipulate the two parts of each baton independently. After training the monkeys, we used single-cell recording to compare neuronal responses to learned and unlearned batons. Responses to learned batons, though not enhanced in strength, were enhanced in selectivity for both individual parts and for whole batons. Whole-baton selectivity arose from a form of conjunctive encoding whereby two parts together exerted a greater influence on neuronal activity than predicted by the additive influence of each part considered individually. These results indicate a possible neural mechanism for holistic or configural effects in expert versus novice observers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wallis, G. & Bülthoff, H. Learning to recognize objects. Trends Cogn. Sci. 3, 22–31 (1999).
Tanaka, J. & Gauthier, I. in Psychology of Learning and Motivation Vol. 36 (eds. Goldstone, R. L., Schyns, P. G. & Medin, D. L.) 83–125 (Academic Press, New York, 1997).
Sheinberg, D.L. & Logothetis, N.K. in Perceptual Learning (eds. Fahle, M. & Poggio, T.) 95–124 (MIT Press, Cambridge, MA, 2002).
Hasegawa, I. & Miyashita, Y. Categorizing the world: expert neurons look into key features. Nat. Neurosci. 5, 90–91 (2002).
Tanaka, K. Inferotemporal cortex and object vision. Annu. Rev. Neurosci. 19, 109–139 (1996).
Logothetis, N.K. & Sheinberg, D.L. Visual object recognition. Annu. Rev. Neurosci. 19, 577–621 (1996).
Baylis, G.C. & Rolls, E.T. Responses of neurons in the inferior temporal cortex in short-term and serial recognition memory tasks. Exp. Brain Res. 65, 614–622 (1987).
Miller, E.K., Li, L. & Desimone, R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science 254, 1377–1379 (1991).
Li, L., Miller, E.K. & Desimone, R. The representation of stimulus familiarity in anterior inferior temporal cortex. J. Neurophysiol. 69, 1918–1929 (1993).
Xiang, J.-Z. & Brown, M.W. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology 37, 657–676 (1998).
Sakai, K. & Miyashita, Y. Neural organization for the long-term memory of paired associates. Nature 354, 152–155 (1991).
Erickson, C.A. & Desimone, R. Responses of macaque perirhinal neurons during and after visual stimulus association learning. J. Neurosci. 19, 10404–10416 (1999).
Messinger, A., Squire, L.R., Zola, S.M. & Albright, T.D. Neuronal representations of stimulus associations develop in the temporal lobe during learning. Proc. Nat. Acad. Sci. USA 98, 12239–12244 (2001).
Logothetis, N.K. & Pauls, J. Psychophysical and physiological evidence for viewer-centered object representations in the primate. Cereb. Cortex 3, 270–288 (1995).
Logothetis, N.K., Pauls, J. & Poggio, T. Shape representation in the inferior temporal cortex of monkeys. Curr. Biol. 5, 552–563 (1995).
Erickson, C.A., Jagadeesh, B. & Desimone, R. Clustering of perirhinal neurons with similar properties following visual experience in adult monkeys. Nat. Neurosci. 3, 1143–1148 (2000).
Vogels, R. & Orban, G.A. Does practice in orientation discrimination lead to changes in the response properties of macaque inferior temporal neurons? Eur. J. Neurosci. 6, 1680–1690 (1994).
Kobatake, E., Wang, G. & Tanaka, K. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J. Neurophysiol. 80, 324–330 (1998).
Sakai, K. & Miyashita, Y. Neuronal tuning to learned complex forms in vision. Neuroreport 5, 829–832 (1994).
Miyashita, Y., Date, A. & Okuno, H. Configurational encoding of complex visual forms by single neurons of monkey temporal cortex. Neuropsychologia 31, 1119–1131 (1993).
Gauthier, I. & Tarr, M.J. Unraveling mechanisms for expert object recognition: bridging brain activity and behavior. J. Exp. Psychol. Hum. Percept. Perform. 28, 431–446 (2002).
Tanaka, J.W. & Farah, M.J. in Analytic and Holistic Processes in Perception of Faces, Objects and Scenes (eds. Peterson, M. A. & Rhodes, G.) (Oxford Univ. Press, New York, in press).
Murray, E.A. & Bussey, T.J. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn. Sci. 3, 142–151 (1999).
Bussey, T.J. & Saksida, L.M. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. Eur. J. Neurosci. 15, 355–364 (2002).
Bussey, T.J., Saksida, L.M. & Murray, E.A. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur. J. Neurosci. 15, 365–374 (2002).
Perrett, D.I., Rolls, E.T. & Caan, W. Visual neurons responsive to faces in the monkey temporal cortex. Exp. Brain Res. 47, 329–342 (1982).
Desimone, R., Albright, T.D., Gross, C.G. & Bruce, C. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci. 4, 2051–2062 (1984).
Tanaka, K., Saito, H., Fukada, Y. & Moriya, M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J. Neurophysiol. 66, 170–189 (1991).
Yamane, S., Kaji, S. & Kawano, K. What facial features activate face neurons in the inferotemporal cortex of the monkey. Exp. Brain Res. 73, 209–214 (1988).
Tsunoda, T., Yamane, Y., Nishizaki, M. & Tanifuji, M. Complex objects are represented in macaque inferotemporal cortex by the combination of feature columns. Nat. Neurosci. 4, 832–838 (2001).
Desimone, R. Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. USA 93, 13494–13499 (1996).
Op De Beeck, H. & Vogels, R. Spatial sensitivity of macaque inferior temporal neurons. J. Comp. Neurol. 426, 505–518 (2000).
Sigala, N. & Logothetis, N.K. Visual categorization shapes feature selectivity in the primate temporal cortex. Nature 415, 318–320 (2002).
Desimone, R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1245–1255 (1998).
Op de Beeck, H., Wagemans, J. & Vogels, R. Inferotemporal neurons represent low-dimensional configurations of parameterized shapes. Nat. Neurosci. 4, 1244–1252 (2001).
Xiang, J.-Z. & Brown, M.W. Differential neuronal responsiveness in primate perirhinal cortex and hippocampal formation during performance of a conditional visual discrimination task. Eur. J. Neurosci. 11, 3715–3724 (1999).
Vogels, R. Categorization of complex visual images by rhesus monkeys. Part 2: single-cell study. Eur. J. Neurosci. 11, 1239–1255 (1999).
Freedman, D.J., Riesenhuber, M., Poggio, T. & Miller, E.K. Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291, 312–316 (2001).
Freedman, D.J., Riesenhuber, M., Poggio, T. & Miller, E.K. Visual categorization and the primate prefrontal cortex: neurophysiology and behavior. J. Neurophysiol. 88, 929–941 (2002).
Goldstone, R.L. Unitization during category learning. J. Exp. Psychol. Hum. Percept. Perform. 26, 86–112 (2000).
Goldstone, R.L. Perceptual learning. Annu. Rev. Psychol. 49, 585–612 (1998).
Zar, J.H. Biostatistical Analysis 4th edn. (Prentice Hall, Upper Saddle River, New Jersey, 1999).
Pasupathy, A. & Connor, C.E. Shape representation in area V4: position-specific tuning for boundary conformation. J. Neurophysiol. 86, 2505–2519 (2001).
Acknowledgements
This work was supported by National Institute of Health grant RO1 EY11831. We thank K. Medler and K. McCracken for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Baker, C., Behrmann, M. & Olson, C. Impact of learning on representation of parts and wholes in monkey inferotemporal cortex. Nat Neurosci 5, 1210–1216 (2002). https://doi.org/10.1038/nn960
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn960
This article is cited by
-
Single-neuron mechanisms of neural adaptation in the human temporal lobe
Nature Communications (2023)
-
Multiple visual objects are represented differently in the human brain and convolutional neural networks
Scientific Reports (2023)
-
Methodological aspects for cognitive architectures construction: a study and proposal
Artificial Intelligence Review (2021)
-
Perirhinal circuits for memory processing
Nature Reviews Neuroscience (2019)
-
Evidence that recurrent circuits are critical to the ventral stream’s execution of core object recognition behavior
Nature Neuroscience (2019)