Abstract
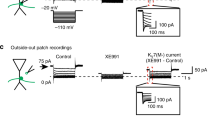
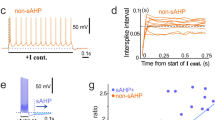
The axon initial segment (AIS) is a specialized region in neurons where action potentials are initiated. It is commonly assumed that this process requires a high density of voltage-gated sodium (Na+) channels. Paradoxically, the results of patch-clamp studies suggest that the Na+ channel density at the AIS is similar to that at the soma and proximal dendrites. Here we provide data obtained by antibody staining, whole-cell voltage-clamp and Na+ imaging, together with modeling, which indicate that the Na+ channel density at the AIS of cortical pyramidal neurons is ∼50 times that in the proximal dendrites. Anchoring of Na+ channels to the cytoskeleton can explain this discrepancy, as disruption of the actin cytoskeleton increased the Na+ current measured in patches from the AIS. Computational models required a high Na+ channel density (∼2,500 pS μm−2) at the AIS to account for observations on action potential generation and backpropagation. In conclusion, action potential generation requires a high Na+ channel density at the AIS, which is maintained by tight anchoring to the actin cytoskeleton.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Coombs, J.S., Curtis, D.R. & Eccles, J.C. The generation of impulses in motoneurones. J. Physiol. (Lond.) 139, 232–249 (1957).
Fatt, P. Sequence of events in synaptic activation of a motoneurone. J. Neurophysiol. 20, 61–80 (1957).
Fuortes, M.G.F., Frank, K. & Becker, M.C. Steps in the production of motoneuron spikes. J. Gen. Physiol. 40, 735–752 (1957).
Eccles, J.C. The Physiology of Synapses (Springer, Berlin, 1964).
Stuart, G.J. & Sakmann, B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367, 69–72 (1994).
Stuart, G. & Hausser, M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron 13, 703–712 (1994).
Colbert, C.M. & Johnston, D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J. Neurosci. 16, 6676–6686 (1996).
Stuart, G., Schiller, J. & Sakmann, B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. (Lond.) 505, 617–632 (1997).
Clark, B.A., Monsivais, P., Branco, T., London, M. & Hausser, M. The site of action potential initiation in cerebellar Purkinje neurons. Nat. Neurosci. 8, 137–139 (2005).
Shu, Y., Duque, A., Yu, Y., Haider, B. & McCormick, D.A. Properties of action-potential initiation in neocortical pyramidal cells: evidence from whole-cell axon recordings. J. Neurophysiol. 97, 746–760 (2007).
Meeks, J.P. & Mennerick, S. Action potential initiation and propagation in CA3 pyramidal axons. J. Neurophysiol. 97, 3460–3472 (2007).
Palmer, L.M. & Stuart, G.J. Site of action potential initiation in layer 5 pyramidal neurons. J. Neurosci. 26, 1854–1863 (2006).
Kole, M.H.P., Letzkus, J.J. & Stuart, G.J. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55, 633–647 (2007).
Dodge, F.A. & Cooley, J.W. Action potential of the motoneuron. IBM J. Res. Develop. 17, 219–229 (1973).
Moore, J.W., Stockbridge, N. & Westerfield, M. On the site of impulse initiation in a neurone. J. Physiol. (Lond.) 336, 301–311 (1983).
Mainen, Z.F., Joerges, J., Huguenard, J.R. & Sejnowski, T.J. A model of spike initiation in neocortical pyramidal neurons. Neuron 15, 1427–1439 (1995).
Rapp, M., Yarom, Y. & Segev, I. Modeling back propagating action potential in weakly excitable dendrites of neocortical pyramidal cells. Proc. Natl. Acad. Sci. USA 93, 11985–11990 (1996).
Wollner, D.A. & Catterall, W.A. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proc. Natl. Acad. Sci. USA 83, 8424–8428 (1986).
Zhou, D. et al. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 143, 1295–1304 (1998).
Kordeli, E., Lambert, S. & Bennett, V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J. Biol. Chem. 270, 2352–2359 (1995).
Komada, M. & Soriano, P. ßIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell Biol. 156, 337–348 (2002).
Boiko, T. et al. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 23, 2306–2313 (2003).
Inda, M.C., DeFelipe, J. & Munoz, A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc. Natl. Acad. Sci. USA 103, 2920–2925 (2006).
Van Wart, A., Trimmer, J.S. & Matthews, G. Polarized distribution of ion channels within microdomains of the axon initial segment. J. Comp. Neurol. 500, 339–352 (2007).
Colbert, C.M. & Pan, E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat. Neurosci. 5, 533–538 (2002).
Ulbricht, W. Sodium channel inactivation: molecular determinants and modulation. Physiol. Rev. 85, 1271–1301 (2005).
Lasser-Ross, N. & Ross, W.N. Imaging voltage and synaptically activated sodium transients in cerebellar Purkinje cells. Proc. Biol. Sci. 247, 35–39 (1992).
Nakada, C. et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 5, 626–632 (2003).
Milton, R.L. & Caldwell, J.H. Na current in membrane blebs: implications for channel mobility and patch clamp recording. J. Neurosci. 10, 885–893 (1990).
Magee, J.C. & Johnston, D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J. Physiol. (Lond.) 487, 67–90 (1995).
Shrager, P. Sodium channels in single demyelinated mammalian axons. Brain Res. 483, 149–154 (1989).
Boiko, T. et al. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30, 91–104 (2001).
Kaplan, M.R. et al. Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron 30, 105–119 (2001).
Rush, A.M., Dib-Hajj, S.D. & Waxman, S.G. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J. Physiol. (Lond.) 564, 803–815 (2005).
Komai, S. et al. Postsynaptic excitability is necessary for strengthening of cortical sensory responses during experience-dependent development. Nat. Neurosci. 9, 1125–1133 (2006).
Peters, A., Proskauer, C.C. & Kaiserman-Abramof, I.R. The small pyramidal neuron of the rat cerebral cortex. The axon hillock and initial segment. J. Cell Biol. 39, 604–619 (1968).
Winckler, B., Forscher, P. & Mellman, I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397, 698–701 (1999).
Garrido, J.J. et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300, 2091–2094 (2003).
Lai, H.C. & Jan, L.Y. The distribution and targeting of neuronal voltage-gated ion channels. Nat. Rev. Neurosci. 7, 548–562 (2006).
Howard, A., Tamas, G. & Soltesz, I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 28, 310–316 (2005).
Kuba, H., Ishii, T.M. & Ohmori, H. Axonal site of spike initiation enhances auditory coincidence detection. Nature 444, 1069–1072 (2006).
Stuart, G.J., Dodt, H.-U. & Sakmann, B. Patch-clamp recordings from the soma and dendrites of neurones in brain slices using infrared video microscopy. Pflugers Arch. 423, 511–518 (1993).
Carnevale, N.T. & Hines, M.L. The Neuron Book (Cambridge University Press, Cambridge, 2006).
Sloper, J.J. & Powell, T.P. A study of the axon initial segment and proximal axon of neurons in the primate motor and somatic sensory cortices. Phil. Trans. R. Soc. Lond. B 285, 173–197 (1979).
Neumcke, B. & Stämpfli, R. Sodium currents and sodium-current fluctuations in rat myelinated nerve fibres. J. Physiol. (Lond.) 329, 163–184 (1982).
Baranauskas, G. & Martina, M. Sodium currents activate without a Hodgkin-and-Huxley-type delay in central mammalian neurons. J. Neurosci. 26, 671–684 (2006).
Taddese, A. & Bean, B.P. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 33, 587–600 (2002).
Canavier, C.C. Sodium dynamics underlying burst firing and putative mechanisms for the regulation of the firing pattern in midbrain dopamine neurons: a computational approach. J. Comput. Neurosci. 6, 49–69 (1999).
Akemann, W. & Knopfel, T. Interaction of Kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of Purkinje neurons. J. Neurosci. 26, 4602–4612 (2006).
Kole, M.H.P., Hallermann, S. & Stuart, G.J. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J. Neurosci. 26, 1677–1687 (2006).
Acknowledgements
We thank W. Catterall for the gift of the pan-alpha sodium channel antibody. This work was supported by the Alexander von Humboldt Foundation (G.J.S.) and a NRSA Senior Fellowship (P.C.R.).
Author information
Authors and Affiliations
Contributions
M.H.P.K. performed the patch and whole-cell current-clamp experiments, as well as simulations, and wrote the paper; S.U.I. performed the antibody experiments; B.M.K. helped with the sodium imaging experiments and performed the associated simulations; S.R.W. and P.C.R. helped with the patch experiments; and G.J.S. performed the whole-cell voltage-clamp and sodium imaging experiments and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Table 1 (PDF 473 kb)
Rights and permissions
About this article
Cite this article
Kole, M., Ilschner, S., Kampa, B. et al. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci 11, 178–186 (2008). https://doi.org/10.1038/nn2040
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2040
This article is cited by
-
A patient-derived mutation of epilepsy-linked LGI1 increases seizure susceptibility through regulating Kv1.1
Cell & Bioscience (2023)
-
Kv7/KCNQ potassium channels in cortical hyperexcitability and juvenile seizure-related death in Ank2-mutant mice
Nature Communications (2023)
-
Projection-Specific Heterogeneity of the Axon Initial Segment of Pyramidal Neurons in the Prelimbic Cortex
Neuroscience Bulletin (2023)
-
Bi-directional Control of Synaptic Input Summation and Spike Generation by GABAergic Inputs at the Axon Initial Segment
Neuroscience Bulletin (2023)
-
Pyramidal cell axon initial segment in Alzheimer´s disease
Scientific Reports (2022)