Abstract
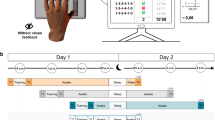
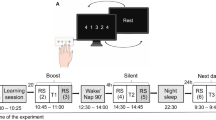
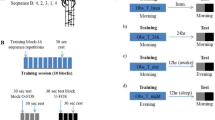
Two behavioral phenomena characterize human motor memory consolidation: diminishing susceptibility to interference by a subsequent experience and the emergence of delayed, offline gains in performance. A recent model proposes that the sleep-independent reduction in interference is followed by the sleep-dependent expression of offline gains. Here, using the finger-opposition sequence–learning task, we show that an interference experienced at 2 h, but not 8 h, following the initial training prevented the expression of delayed gains at 24 h post-training. However, a 90-min nap, immediately post-training, markedly reduced the susceptibility to interference, with robust delayed gains expressed overnight, despite interference at 2 h post-training. With no interference, a nap resulted in much earlier expression of delayed gains, within 8 h post-training. These results suggest that the evolution of robustness to interference and the evolution of delayed gains can coincide immediately post-training and that both effects reflect sleep-sensitive processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karni, A. & Sagi, D. The time course of learning a visual skill. Nature 365, 250–252 (1993).
Brashers-Krug, T., Shadmehr, R. & Bizzi, E. Consolidation in human motor memory. Nature 382, 252–255 (1996).
Walker, M.P. A refined model of sleep and the time course of memory formation. Behav. Brain Sci. 28, 51–64 discussion 64–104 (2005).
Stickgold, R., James, L. & Hobson, J.A. Visual discrimination learning requires sleep after training. Nat. Neurosci. 3, 1237–1238 (2000).
Fischer, S., Hallschmid, M., Elsner, A.L. & Born, J. Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. USA 99, 11987–11991 (2002).
Maquet, P. The role of sleep in learning and memory. Science 294, 1048–1052 (2001).
Robertson, E.M, Pascual-Leone, A. & Miall, R.C. Current concepts in procedural consolidation. Nat. Rev. Neurosci. 5, 576–582 (2004).
Karni, A. et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc. Natl. Acad. Sci. USA 95, 861–868 (1998).
Korman, M., Raz, N., Flash, T. & Karni, A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc. Natl. Acad. Sci. USA 100, 12492–12497 (2003).
Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 (2004).
McGaugh, J.L. Memory—a century of consolidation. Science 287, 248–251 (2000).
Adams, J.A. Historical review and appraisal of research on the learning, retention, and transfer of human motor skills. Psychol. Bull. 101, 41–74 (1987).
Walker, M.P. & Stickgold, R. Sleep–dependent learning and memory consolidation. Neuron 44, 121–133 (2004).
Luft, A.R., Buitrago, M.M., Ringer, T., Dichgans, J. & Schulz, J.B. Motor skill learning depends on protein synthesis in motor cortex after training. J. Neurosci. 24, 6515–6520 (2004).
Luft, A.R. & Buitrago, M.M. Stages of motor skill learning. Mol. Neurobiol. 32, 205–216 (2005).
Hauptmann, B., Reinhart, E., Brandt, S.A. & Karni, A. The predictive value of the leveling off of within session performance for procedural memory consolidation. Brain Res. Cogn. Brain Res. 24, 181–189 (2005).
Roth, D.A., Kishon-Rabin, L., Hildesheimer, M. & Karni, A. A latent consolidation phase in auditory identification learning, time in the awake state is sufficient. Learn. Mem. 12, 159–164 (2005).
Gais, S., Plihal, W., Wagner, U. & Born, J. Early sleep triggers memory for early visual discrimination skills. Nat. Neurosci. 3, 1335–1339 (2000).
Fischer, S., Nitschke, M.F., Melchert, U.H., Erdmann, C. & Born, J. Motor memory consolidation in sleep shapes more effective neuronal representations. J. Neurosci. 25, 11248–11255 (2005).
Maquet, P., Schwartz, S., Passingham, R. & Frith, C. Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J. Neurosci. 23, 1432–1440 (2003).
Walker, M.P., Brakefield, T., Hobson, J.A. & Stickgold, R. Dissociable stages of human memory consolidation and reconsolidation. Nature 425, 616–620 (2003).
Walker, M.P. et al. Sleep and the time course of motor skill learning. Learn. Mem. 10, 275–284 (2003).
Karni, A. The acquisition of perceptual and motor skills, a memory system in the adult human cortex. Brain Res. Cogn. Brain Res. 5, 39–48 (1996).
Walker, M.P., Brakefield, T., Morgan, A., Hobson, J.A. & Stickgold, R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35, 205–211 (2002).
Shadmehr, R. & Holcomb, H.H. Neural correlates of motor memory consolidation. Science 277, 821–825 (1997).
Walker, M.P. & Stickgold, R. Sleep, memory and plasticity. Annu. Rev. Psychol. 57, 139–166 (2006).
Muellbacher, W. et al. Early consolidation in human primary motor cortex. Nature 415, 640–644 (2002).
Ungerleider, L.G., Doyon, J. & Karni, A. Imaging brain plasticity during motor skill learning. Neurobiol. Learn. Mem. 78, 553–564 (2002).
Walker, M.P. & Stickgold, R. It's practice with sleep that makes perfect: implications of sleep-dependent learning and plasticity for skill performance. Clin. Sports Med. 24, 301–317 (2005).
Goedert, K.M. & Willingham, D.B. Patterns of interference in sequence learning and prism adaptation inconsistent with the consolidation hypothesis. Learn. Mem. 9, 279–292 (2002).
Mednick, S., Nakayama, K. & Stickgold, R. Sleep-dependent learning: a nap is as good as a night. Nat. Neurosci. 6, 697–698 (2003).
Robertson, E.M., Pascual-Leone, A. & Press, D.Z. Awareness modifies the skill-learning benefits of sleep. Curr. Biol. 14, 208–212 (2004).
Peigneux, P. et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 44, 535–545 (2004).
Rauchs, G., Desgranges, B., Foret, J. & Eustache, F. The relationships between memory systems and sleep stages. J. Sleep Res. 14, 123–140 (2005).
Karni, A., Tanne, D., Rubenstein, B.S., Askenasy, J.J. & Sagi, D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265, 679–682 (1994).
Plihal, W. & Born, J. Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 9, 534–547 (1997).
Vertes, R.P. Memory consolidation in sleep; dream or reality. Neuron 44, 135–148 (2004).
Krakauer, J.W. & Shadmehr, R. Consolidation of motor memory. Trends Neurosci. 29, 58–64 (2006).
Cohen, D.A., Pascual-Leone, A., Press, D.Z. & Robertson, E.M. Offline learning of motor skill memory: a double dissociation of goal and movement. Proc. Natl. Acad. Sci. USA 102, 18237–18241 (2005).
Hikosaka, O. et al. Parallel neural networks for learning sequential procedures. Trends Neurosci. 22, 464–471 (1999).
Nudo, R.J., Milliken, G.W., Jenkins, W.M. & Merzenich, M.M. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J. Neurosci. 16, 785–807 (1996).
Doyon, J., Penhune, V. & Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41, 252–262 (2003).
Sosnik, R., Hauptmann, B., Karni, A. & Flash, T. When practice leads to co-articulation: the evolution of geometrically defined movement primitives. Exp. Brain Res. 156, 422–438 (2004).
Orban, P. et al. Sleep after spatial learning promotes covert reorganization of brain activity. Proc. Natl. Acad. Sci. USA 103, 7124–7129 (2006).
Korman, M., Flash, T. & Karni, A. Synaptic and systems consolidation may explain the complex relationship between resistance to interference and the emergence of delayed gains in newly acquired procedural memories. Behav. Brain Sci. 28, 51–64 discussion 64–104 (2005).
Censor, N., Karni, A. & Sagi, D. A link between perceptual learning, adaptation and sleep. Vision Res. 46, 4071–4074 (2006).
Tononi, G. & Cirelli, C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62 (2006).
Turrigiano, G.G. & Nelson, S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107 (2004).
Oldfield, R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Rechtschaffen, A. & Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. (U.S. Government Printing Office, Washington, DC, 1968).
Acknowledgements
Support for this research was provided by the Council of Higher Education in Israel (M.K.), Israel Science Foundation (Y.D. and A.K.) and the Canadian Institutes of Health Research (J. Doyon, J.C. and A.K.).
Author information
Authors and Affiliations
Contributions
M.K. designed and conducted the experiments and data analysis and wrote the manuscript; J. Doyon provided theoretical input and edited the manuscript; J. Doljansky analyzed sleep recordings; J.C. provided technical input and edited the manuscript; Y.D. supervised the sleep-related aspects of the project; A.K. designed and supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Korman, M., Doyon, J., Doljansky, J. et al. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci 10, 1206–1213 (2007). https://doi.org/10.1038/nn1959
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1959
This article is cited by
-
Motor learning- and consolidation-related resting state fast and slow brain dynamics across wake and sleep
Scientific Reports (2024)
-
Sequence-specific delayed gains in motor fluency evolve after movement observation training in the absence of early sleep
Scientific Reports (2024)
-
Generalization of procedural motor sequence learning after a single practice trial
npj Science of Learning (2023)
-
Early sleep after action observation and motor imagery training boosts improvements in manual dexterity
Scientific Reports (2023)
-
Time-of-day effects on skill acquisition and consolidation after physical and mental practices
Scientific Reports (2022)