Abstract
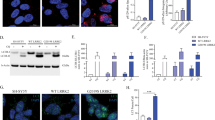
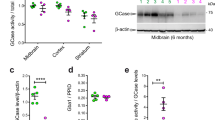
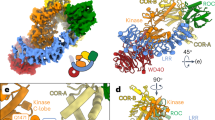
Mutations in the the leucine-rich repeat kinase-2 (LRRK2) gene cause autosomal-dominant Parkinson disease and some cases of sporadic Parkinson disease. Here we found that LRRK2 kinase activity was regulated by GTP via the intrinsic GTPase Roc domain, and alterations of LRRK2 protein that reduced kinase activity of mutant LRRK2 correspondingly reduced neuronal toxicity. These data elucidate the pathogenesis of LRRK2-linked Parkinson disease, potentially illuminate mechanisms of sporadic Parkinson disease and suggest therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Paisan-Ruiz, C. et al. Neuron 44, 595–600 (2004).
Zimprich, A. et al. Neuron 44, 601–607 (2004).
Di Fonzo, A. et al. Lancet 365, 412–415 (2005).
Gilks, W.P. et al. Lancet 365, 415–416 (2005).
Morris, H.R. Ann. Med. 37, 86–96 (2005).
Gasser, T. Curr. Opin. Neurol. 18, 363–369 (2005).
Farrer, M. et al. Neurology. 65, 738–740 (2005).
Albrecht, M. Lancet 365, 1230 (2005).
West, A.B. et al. Proc. Natl. Acad. Sci. USA 102, 16842–16847 (2005).
Gloeckner, C.J. et al. Hum. Mol. Genet. 15, 223–232 (2006).
Greggio, E. et al. Neurobiol. Dis. 23, 329–341 (2006).
Smith, W.W. et al. Proc. Natl. Acad. Sci. USA 102, 18676–18681 (2005).
Bosgraaf, L. & van Haastert, P.J. Biochim. Biophys. Acta 1643, 5–10 (2003).
Korr, D. et al. Cell. Signal. 18, 910–920 (2006).
Praefcke, G.J. et al. J. Mol. Biol. 344, 257–269 (2004).
Acknowledgements
This work was supported by the US National Institutes of Health, the National Institute of Neurological Disorders and Stroke (R21NS055684-01 and NS38377) and the National Parkinson's Disease Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Smith, W., Pei, Z., Jiang, H. et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 9, 1231–1233 (2006). https://doi.org/10.1038/nn1776
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1776
This article is cited by
-
Role of neuroinflammation in neurodegeneration development
Signal Transduction and Targeted Therapy (2023)
-
The use of fibroblasts as a valuable strategy for studying mitochondrial impairment in neurological disorders
Translational Neurodegeneration (2022)
-
LRRK2 mutant knock-in mouse models: therapeutic relevance in Parkinson's disease
Translational Neurodegeneration (2022)
-
Inflammation and immune dysfunction in Parkinson disease
Nature Reviews Immunology (2022)
-
The role of tyrosine hydroxylase–dopamine pathway in Parkinson’s disease pathogenesis
Cellular and Molecular Life Sciences (2022)