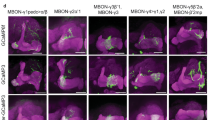
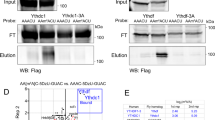
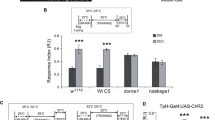
Abstract
Heterotrimeric G(o) is one of the most abundant proteins in the brain, yet relatively little is known of its neural functions in vivo. Here we demonstrate that G(o) signaling is required for the formation of associative memory. In Drosophila melanogaster, pertussis toxin (PTX) is a selective inhibitor of G(o) signaling. The postdevelopmental expression of PTX within mushroom body neurons robustly and reversibly inhibits associative learning. The effect of G(o) inhibition is distributed in both γ- and α/β-lobe mushroom body neurons. However, the expression of PTX in neurons adjacent to the mushroom bodies does not affect memory. PTX expression also does not interact genetically with a rutabaga adenylyl cyclase loss-of-function mutation. Thus, G(o) defines a new signaling pathway required in mushroom body neurons for the formation of associative memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tully, T. & Quinn, W.G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. [A] 157, 263–277 (1985).
Gerber, B., Tanimoto, H. & Heisenberg, M. Evaluating the evidence from fruit flies. Curr. Opin. Neurobiol. 14, 737–744 (2004).
Dudai, Y., Jan, Y.N., Byers, D., Quinn, W.G. & Benzer, S. dunce, a mutant of Drosophila deficient in learning. Proc. Natl. Acad. Sci. USA 73, 1684–1689 (1976).
Byers, D., Davis, R.L. & Kiger, J.A. Jr. Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature 289, 79–81 (1981).
Skoulakis, E.M., Kalderon, D. & Davis, R.L. Preferential expression of the catalytic subunit of PKA in the mushroom bodies and its role in learning and memory. Neuron 11, 197–208 (1993).
Goodwin, S.F. et al. Defective learning in mutants of the Drosophila gene for a regulatory subunit of cAMP-dependent protein kinase. J. Neurosci. 17, 8817–8827 (1997).
Connolly, J.B. et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science 274, 2104–2107 (1996).
Zars, T., Fischer, M., Schulz, R. & Heisenberg, M. Localization of a short-term memory in Drosophila. Science 288, 672–675 (2000).
McGuire, S.E., Le, P.T., Osborn, A.J., Matsumoto, K. & Davis, R.L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768 (2003).
Mao, Z., Roman, G., Zong, L. & Davis, R.L. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc. Natl. Acad. Sci. USA 101, 198–203 (2004).
Sternweis, P.C. & Robishaw, J.D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J. Biol. Chem. 259, 13806–13813 (1984).
Jiang, M. et al. Multiple neurological abnormalities in mice deficient in the G protein Go . Proc. Natl. Acad. Sci. USA 95, 3269–3274 (1998).
Mendel, J.E. et al. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science 267, 1652–1655 (1995).
Frémion, F. et al. The heterotrimeric protein Go is required for the formation of heart epithelium in Drosophila. J. Cell Biol. 145, 1063–1076 (1999).
Thambi, N.C., Quan, F., Wolfgang, W.J., Spiegel, A. & Forte, M. Immunological and molecular characterization of Go alpha-like proteins in the Drosophila central nervous system. J. Biol. Chem. 264, 18552–18560 (1989).
Guillen, A. et al. A Go-like protein in Drosophila melanogaster and its expression in memory mutants. EMBO J. 9, 1449–1455 (1990).
Katada, T., Tamura, M. & Ui, M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch. Biochem. Biophys. 224, 290–298 (1983).
Hopkins, R.S., Stamnes, M.A., Simon, M.I. & Hurley, J.B. Cholera toxin and pertussis toxin substrates and endogenous ADP-ribosyltransferase activity in Drosophila melanogaster. Biochim. Biophys. Acta 970, 355–362 (1988).
Fitch, C.L. et al. Pertussis toxin expression in Drosophila alters the visual response and blocks eating behaviour. Cell. Signal. 5, 187–207 (1993).
Osterwalder, T., Yoon, K.S., White, B.H. & Keshishian, H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA 98, 12596–12601 (2001).
Schwaerzel, M., Heisenberg, M. & Zars, T. Extinction antagonizes olfactory memory at the subcellular level. Neuron 35, 951–960 (2002).
Crittenden, J.R., Skoulakis, E.M., Han, K.A., Kalderon, D. & Davis, R.L. Tripartite mushroom body architecture revealed by antigenic markers. Learn. Mem. 5, 38–51 (1998).
Waddell, S., Armstrong, J.D., Kitamoto, T., Kaiser, K. & Quinn, W.G. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 103, 805–813 (2000).
Dubnau, J., Grady, L., Kitamoto, T. & Tully, T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 411, 476–480 (2001).
Isabel, G., Pascual, A. & Preat, T. Exclusive consolidated memory phases in Drosophila. Science 304, 1024–1027 (2004).
Strausfeld, N.J., Sinakevitch, I. & Vilinsky, I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc. Res. Tech. 62, 151–169 (2003).
Taussig, R., Tang, W.J., Hepler, J.R. & Gilman, A.G. Distinct patterns of bidirectional regulation of mammalian adenylyl cyclases. J. Biol. Chem. 269, 6093–6100 (1994).
Sharma, S.K., Klee, W.A. & Nirenberg, M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc. Natl. Acad. Sci. USA 72, 3092–3096 (1975).
Ikeda, S.R. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380, 255–258 (1996).
Roman, G., He, J. & Davis, R.L. A new series of Drosophila expression vectors suitable for behavioral rescue. Biotechniques 27, 54–56 (1999).
Acknowledgements
The authors wish to thank J. Maynard (Stanford University, Palo Alto, California) for providing the 1B7 monoclonal antibody to PTX, and R. Davis, K. Choi and M. Mancini for sharing space in their laboratories. This work was supported by NS042185-04 awarded to G.R.
Author information
Authors and Affiliations
Contributions
The behavioral experiments were performed by both J.F. and H.G. H.G. performed the immunohistochemistry and L.L. performed the western blot analysis. G.R. generated the PTX transgene and the tested genotypes.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Lethality of P{UASPTX} Expression. (PDF 50 kb)
Supplementary Table 2
Naïve odour and shock avoidance in a 2-minute T-Maze assay. (PDF 78 kb)
Rights and permissions
About this article
Cite this article
Ferris, J., Ge, H., Liu, L. et al. G(o) signaling is required for Drosophila associative learning. Nat Neurosci 9, 1036–1040 (2006). https://doi.org/10.1038/nn1738
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1738
This article is cited by
-
Descending GABAergic pathway links brain sugar-sensing to peripheral nociceptive gating in Drosophila
Nature Communications (2023)
-
Muscarinic acetylcholine receptor signaling generates OFF selectivity in a simple visual circuit
Nature Communications (2019)
-
Drosophila melanogaster positive transcriptional elongation factors regulate metabolic and sex-biased expression in adults
BMC Genomics (2017)
-
Operation of a homeostatic sleep switch
Nature (2016)
-
TORC2: a novel target for treating age-associated memory impairment
Scientific Reports (2015)