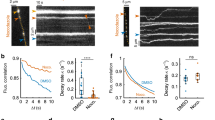
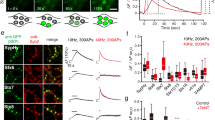
Abstract
Synapses require resources synthesized in the neuronal soma, but there are no known mechanisms to overcome delays associated with the synthesis and axonal transport of new proteins generated in response to activity, or to direct resources specifically to active synapses. Here, in vivo imaging of the Drosophila melanogaster neuromuscular junction reveals a cell-biological strategy that addresses these constraints. Peptidergic vesicles continually transit through resting terminals, but retrograde peptidergic vesicle flux is accessed following activity to rapidly boost neuropeptide content in synaptic boutons. The presence of excess transiting vesicles implies that synaptic neuropeptide stores are limited by the capture of peptidergic vesicles at the terminal, rather than by synthesis in the soma or delivery via the axon. Furthermore, activity-dependent capture from a pool of transiting vesicles provides a nerve terminal–based mechanism for directing distally and slowly generated resources quickly to active synapses. Finally, retrograde transport in the nerve terminal is regulated by activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zupanc, G.K. Peptidergic transmission: from morphological correlates to functional implications. Micron 27, 35–91 (1996).
Burbach, J.P., Luckman, S.M., Murphy, D. & Gainer, H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 81, 1197–1267 (2001).
Taghert, P.H. & Veenstra, J.A. Drosophila neuropeptide signaling. Adv. Genet. 49, 1–65 (2003).
Nassel, D.R. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog. Neurobiol. 68, 1–84 (2002).
Ahmari, S.E., Buchanan, J. & Smith, S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3, 445–451 (2000).
Zhai, R.G. et al. Assembling the presynaptic active zone: a characterization of an active zone precursor vesicle. Neuron 29, 131–143 (2001).
Lessmann, V., Gottmann, K. & Malcangio, M. Neurotrophin secretion: current facts and future prospects. Prog. Neurobiol. 69, 341–374 (2003).
Pierce, J.P. & Milner, T.A. Parallel increases in synaptic and surface areas of mossy fiber terminals following seizure induction. Synapse 39, 249–256 (2001).
Murthy, V.N., Schikorski, T., Stevens, C.F. & Zhu, Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32, 673–682 (2001).
Brown, A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J. Cell Biol. 160, 817–821 (2003).
Shakiryanova, D., Tully, A., Hewes, R.S., Deitcher, D.L. & Levitan, E.S. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat. Neurosci. 8, 173–178 (2005).
Rao, S., Lang, C., Levitan, E.S. & Deitcher, D.L. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 49, 159–172 (2001).
Heifetz, Y. & Wolfner, M.F. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc. Natl. Acad. Sci. USA 101, 6261–6266 (2004).
Husain, Q.M. & Ewer, J. Use of targetable GFP-tagged neuropeptide for visualizing neuropeptide release following execution of a behavior. J. Neurobiol. 59, 181–191 (2004).
Mudher, A. et al. GSK-3β inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psych. 9, 522–530 (2004).
Sturman, D.A, Shakiryanova, D., Hewes, R.S., Deitcher, D.L. & Levitan, E.S. Nearly neutral secretory vesicles in Drosophila nerve terminals. Biophys. J. 90, L45–L47 (2006).
Atwood, H.L., Govind, C.K. & Wu, C.F. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J. Neurobiol. 24, 1008–1024 (1993).
Jia, X.X., Gorczyca, M. & Budnik, V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J. Neurobiol. 24, 1025–1044 (1993).
Levitan, E.S. Studying neuronal peptide release and secretory granule dynamics with green fluorescent protein. Methods 16, 182–187 (1998).
Krueger, S.R., Kolar, A. & Fitzsimonds, R.M. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron 40, 945–957 (2003).
Darcy, K.J., Staras, K., Collinson, L.M. & Goda, Y. Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat. Neurosci. 9, 315–321 (2006).
Neubig, R. The time course of drug action. in Principles of Drug Action. 3rd edn. (eds. Pratt, W.B. & Taylor, P.) 311–313 (Churchill-Livingstone, London, 1990).
Acknowledgements
We thank W.C. DeGroat and K. Kandler for their comments. This research was supported by grant NS32385 from the US National Institutes of Health to E.S.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Constitutive replacement of synaptic DCVs is slow. (PDF 137 kb)
Supplementary Fig. 2
Detection of vesicle shipments. (PDF 73 kb)
Supplementary Fig. 3
Movement of vesicle shipments. (PDF 189 kb)
Rights and permissions
About this article
Cite this article
Shakiryanova, D., Tully, A. & Levitan, E. Activity-dependent synaptic capture of transiting peptidergic vesicles. Nat Neurosci 9, 896–900 (2006). https://doi.org/10.1038/nn1719
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1719
This article is cited by
-
Neurotrophic factors and target-specific retrograde signaling interactions define the specificity of classical and neuropeptide cotransmitter release at identified Lymnaea synapses
Scientific Reports (2020)
-
A fast and robust method for automated analysis of axonal transport
European Biophysics Journal (2011)
-
The essential role of bursicon during Drosophiladevelopment
BMC Developmental Biology (2010)
-
Dynamics of peptidergic secretory granule transport are regulated by neuronal stimulation
BMC Neuroscience (2010)
-
Dominant-Negative Myosin Va Impairs Retrograde but Not Anterograde Axonal Transport of Large Dense Core Vesicles
Cellular and Molecular Neurobiology (2010)