Abstract
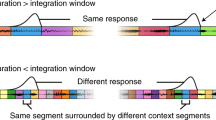
Lateralization of function in auditory cortex has remained a persistent puzzle. Previous studies using signals with differing spectrotemporal characteristics support a model in which the left hemisphere is more sensitive to temporal and the right more sensitive to spectral stimulus attributes. Here we use single-trial sparse-acquisition fMRI and a stimulus with parametrically varying segmental structure affecting primarily temporal properties. We show that both left and right auditory cortices are remarkably sensitive to temporal structure. Crucially, beyond bilateral sensitivity to timing information, we uncover two functionally significant interactions. First, local spectrotemporal signal structure is differentially processed in the superior temporal gyrus. Second, lateralized responses emerge in the higher-order superior temporal sulcus, where more slowly modulated signals preferentially drive the right hemisphere. The data support a model in which sounds are analyzed on two distinct timescales, 25–50 ms and 200–300 ms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hall, D.A., Hart, H.C. & Johnsrude, I.S. Relationships between human auditory cortical structure and function. Audiol. Neurootol. 8, 1–18 (2003).
Zatorre, R., Belin, P. & Penhune, V. Structure and function of auditory cortex: music and speech. Trends Cogn. Sci. 6, 37–46 (2002).
Poeppel, D. The analysis of speech in different temporal integration windows: cerebral lateralization as 'asymmetric sampling in time'. Speech Commun. 41, 245–255 (2003).
Zatorre, R. & Belin, P. Spectral and temporal processing in human auditory cortex. Cereb. Cortex 11, 946–953 (2001).
Binder, J.R. et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex 10, 512–528 (2000).
Scott, S.K. & Johnsrude, I.S. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 26, 100–107 (2003).
Hickok, G. & Poeppel, D. Towards a functional neuroanatomy of speech perception. Trends Cogn. Sci. 4, 131–138 (2000).
Johnsrude, I.S., Penhune, V.B. & Zatorre, R.J. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 123, 155–163 (2000).
Scott, S.K., Blank, C.C., Rosen, S. & Wise, R.J. Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123, 2400–2406 (2000).
Gandour, J. et al. A cross-linguistic FMRI study of spectral and temporal cues underlying phonological processing. J. Cogn. Neurosci. 14, 1076–1087 (2002).
Giraud, A-L. et al. Representation of the temporal envelope of sounds in the human brain. J. Neurophysiol. 84, 1588–1598 (2000).
Shamma, S. On the role of space and time in auditory processing. Trends Cogn. Sci. 5, 340–348 (2001).
Hall, D.A. et al. Spectral and temporal processing in human auditory cortex. Cereb. Cortex 12, 140–149 (2002).
Hall, D.A. et al. 'Sparse' temporal sampling in auditory fMRI. Hum. Brain Mapp. 7, 213–223 (1999).
Edmister, W.B., Talavage, T.M., Ledden, P.J. & Weisskoff, R.M. Improved auditory cortex imaging using clustered volume acquisitions. Hum. Brain Mapp. 7, 89–97 (1999).
Stevens, K.N. Acoustic Phonetics (MIT Press, Cambridge, Massachusetts, USA, 1998).
Moore, B.C.J. in Human Psychophysics (eds. Yost, W.A., Popper, A.N. & Fay, R.R.) (Springer, New York, 1993).
Hackett, T.A., Preuss, T.M. & Kaas, J.H. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J. Comp. Neurol. 441, 197–222 (2001).
Griffiths, T.D., Buchel, C., Frackowiak, R.S. & Patterson, R.D. Analysis of temporal structure in sound by the human brain. Nat. Neurosci. 1, 422–427 (1998).
Harms, M.P. & Melcher, J.R. Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J. Neurophysiol. 88, 1433–1450 (2002).
Wang, X., Lu, T. & Liang, L. Cortical processing of temporal modulations. Speech Commun. 41, 107–121 (2003).
Wessinger, C.M. et al. Hierarchical organization of the human auditory cortex revealed by functional magnetic resonance imaging. J. Cogn. Neurosci. 13, 1–7 (2001).
Yost, W.A. Auditory image perception and analysis: the basis for hearing. Hear. Res. 56, 8–18 (1991).
Yabe, H. et al. Organizing sound sequences in the human brain: the interplay of auditory streaming and temporal integration. Brain Res. 897, 222–227 (2001).
Winkler, I., Reinikainen, K. & Naatanen, R. Event-related brain potentials reflect traces of echoic memory in humans. Percept. Psychophys. 53, 443–449 (1993).
Sussman, E., Winkler, I., Ritter, W., Alho, K. & Naatanen, R. Temporal integration of auditory stimulus deviance as reflected by the mismatch negativity. Neurosci. Lett. 264, 161–164 (1999).
Zwislocki, J. Theory of temporal auditory summation. J. Acoust. Soc. Am. 32, 1046–1060 (1960).
Kaas, J.H. & Hackett, T.A. Subdivisions of auditory cortex and processing streams in primates. Proc. Natl Acad. Sci. USA 97, 11793–11799 (2000).
Geschwind, N. & Levitsky, W. Human brain: left-right asymmetries in temporal speech region. Science 161, 186–187 (1968).
Galuske, R.A., Schlote, W., Bratzke, H. & Singer, W. Interhemispheric asymmetries of the modular structure in human temporal cortex. Science 289, 1946–1949 (2000).
Jäncke, L., Wustenberg, T., Scheich, H. & Heinze, H.J. Phonetic perception and the temporal cortex. Neuroimage 15, 733–746 (2002).
Näätänen, R. et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature 385, 432–434 (1997).
Palva, S. et al. Distinct gamma-band evoked responses to speech and non-speech sounds in humans. J. Neurosci. 22, RC211 (2002).
Schwartz, J. & Tallal, P. Rate of acoustic change may underlie hemispheric specialization for speech perception. Science 207, 1380–1381 (1980).
Divenyi, P.L. & Robinson, A.J. Nonlinguistic auditory capabilities in aphasia. Brain Lang. 37, 290–326 (1989).
Meyer, M., Alter, K., Friederici, A.D., Lohmann, G. & von Cramon, D.Y. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum. Brain Mapp. 17, 73–88 (2002).
Ivry, R.B. & Robertson, L.C. The Two Sides of Perception (Bradford Books, MIT Press, Cambridge, Massachusetts, 1997).
Lu, T., Liang, L. & Wang, X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat. Neurosci. 4, 1131–1138 (2001).
Smith, Z.M., Delgutte, B. & Oxenham, A.J. Chimaeric sounds reveal dichotomies in auditory perception. Nature 416, 87–90 (2002).
Acknowledgements
We thank P. Bandettini, J. Fritz, A.-L. Giraud, A. Martin and J. Rauschecker for insightful critical comments; F. Husain for help with experimental setup; and K.M. Boemio for her continued and continuous encouragement. A.B. and D.P. were supported by US National Institutes of Health R01 DC05660 to D.P. During the preparation of the manuscript, D.P. was a fellow at the Wissenschaftskolleg zu Berlin and the American Academy Berlin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
STS activation from single-subject ROI analysis. (PDF 102 kb)
Supplementary Fig. 2
Differential cortical connectivity model depicting hypothesized connectivity between areas STG and STS. (PDF 25 kb)
Supplementary Audio 1
CN (WAV 775 kb)
Supplementary Audio 2
FM, 25 ms (WAV 775 kb)
Supplementary Audio 3
FM, 300 ms (WAV 775 kb)
Supplementary Audio 4
TN, 25 ms (WAV 775 kb)
Supplementary Audio 5
TN, 300 ms (WAV 775 kb)
Rights and permissions
About this article
Cite this article
Boemio, A., Fromm, S., Braun, A. et al. Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci 8, 389–395 (2005). https://doi.org/10.1038/nn1409
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1409
This article is cited by
-
Spectrotemporal content of human auditory working memory represented in functional connectivity patterns
Communications Biology (2023)
-
Loudness affects motion: asymmetric volume of auditory feedback results in asymmetric gait in healthy young adults
BMC Musculoskeletal Disorders (2022)
-
Amplitude and frequency modulation of subthalamic beta oscillations jointly encode the dopaminergic state in Parkinson’s disease
npj Parkinson's Disease (2022)
-
Side-of-Implantation Effect on Functional Asymmetry in the Auditory Cortex of Single-Sided Deaf Cochlear-Implant Users
Brain Topography (2022)
-
Neural Mechanisms Underlying Human Auditory Evoked Responses Revealed By Human Neocortical Neurosolver
Brain Topography (2022)