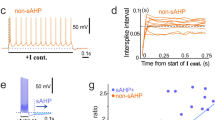
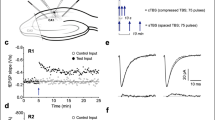
Abstract
Although kainate receptor activation has been known to evoke epileptiform activity, little is known about the role of kainate receptors in synaptic transmission. Here we report that kainate (KA) receptors are present on interneurons and, when activated, cause a large increase in the frequency of spontaneous inhibitory postsynaptic currents (IPSCs) driven by action potentials. Stimulation of excitatory afferents generates a pharmacologically identifiable synaptic current mediated by KA receptors in interneurons. This synaptic current is similar to that mediated by AMPA receptors in its response to short stimulus trains, current–voltage relations and coefficient of variation, but it is much smaller in peak amplitude and much slower. KA application also considerably depresses evoked IPSCs. This depression seems to be in large part an indirect consequence of the repetitive firing evoked by the activation of the interneuronal somatic/dendritic KA receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hollmann, M. & Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31– 108 (1994).
Watkins, J. C. & Evans, R. H. Excitatory amino acid transmitters . Annu. Rev. Pharmacol. Toxicol. 21, 165 –204 (1981).
Sommer, B. & Seeburg, P. H. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol. Sci. 13, 291–296 (1992).
Bettler, B. & Mulle, C. Review: Neurotransmitter receptors II. AMPA and kainate receptors. Neuropharmacology 34 , 123–139 (1995).
Mayer, M. Kainate receptors. Finding homes at synapses. Nature 389, 542–543 (1997).
Lerma,J. Kainate reveals its targets. Neuron 19, 1155– 1158 (1997).
Paternain, A. V., Morales, M. & Lerma, J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14, 185–189 (1995).
Huettner, J. E. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by ConA. Neuron 5, 255–266 (1990).
Lerma, J., Paternain, A. V., Naranjo, J. R. & Mellstrom, B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons . Proc. Natl. Acad. Sci. USA 90, 11688– 11692 (1993).
Ruano, D., Lambolez, B., Rossier, J., Paternain, A. V. & Lerma, J. Kainate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neuron 14, 1009– 1017 (1995).
Wilding, T. J. & Huettner, J. E. Activation and desensitization of hippocampal kainate receptors. J. Neurosci. 17, 2713–2721 (1997).
Patneau, D. K., Wright, P. W., Winters, C., Mayer, M. L. & Gallo, V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor . Neuron 12, 357–371 (1994).
Castillo, P. E., Malenka, R. C. & Nicoll, R. A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186 (1997).
Vignes, M. & Collingridge, G. L. The synaptic activation of kainate receptors. Nature 388, 179– 182 (1997).
Chittajallu, R. et al. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379, 78– 81 (1996).
Clarke, V. R. et al. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389, 599– 603 (1997).
Rodriguez-Moreno, A., Herreras, O. & Lerma, J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19, 893–901 (1997).
Rodriguez-Moreno, A. & Lerma, J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20, 1211–1218 (1998).
Alger, B. E. & Nicoll, R. A. Spontaneous inhibitory postsynaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 200, 195–200 (1980).
Alger, B. E. et al. Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J. Physiol. (Lond.) 496, 197–209 (1996).
Otis, T. S., Staley, K. J. & Mody, I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 545 , 142–150 (1991).
Wilding, T. J. & Huettner, J. E. Differential antagonism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol. Pharmacol. 47, 582–587 (1995).
Burnashev, N., Villarroel, A. & Sakmann, B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J. Physiol. 496, 165–173 (1996).
Scanziani, M., Salin, P. A., Vogt, K. E., Malenka, R. C. & Nicoll, R. A. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature 385, 630–634 (1997).
Howe, J. R. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J. Neurophysiol. 76, 510–519 (1996).
Swanson, G. T., Feldmeyer, D., Kaneda, M. & Cull-Candy, S. G. Effect of RNA editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. J. Physiol. (Lond.) 492, 129–142 (1996).
Jones, M. V. & Westbrook, G. L. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron 15, 181–191 (1995).
Kamiya, H., & Ozawa, S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J. Physiol. (Lond.) 509, 833 –845 (1998).
Robinson, J. H. & Deadwyler, S. A. Kainic acid produces depolarisation of CA3 pyramidal cells in the in vitro hippocampal slice. Brain Res. 221, 117– 127 (1981).
Westbrook, G. L. & Lothman, E. W. Cellular and synaptic basis of kainic acid-induced hippocampal epileptiform activity. Brain Res. 273, 97–109 (1983).
Fisher, R. S. & Alger, B. E. Electrophysiological mechanisms of kainic acid-induced epileptiform activity in the rat hippocampal slice . J. Neurosci. 4, 1312– 1323 (1984).
Cornish, S. M. & Wheal, H. V. Long-term loss of paired pulse inhibition in the kainic acid-lesioned hippocampus of the rat. Neuroscience 28, 563– 571 (1989).
Lancaster, B. & Wheal, H. V. Chronic failure of inhibition of the CA1 area of the hippocampus following kainic acid lesions of the CA3/4 area. Brain Res. 295, 317– 324 (1984).
Franck, FNM>J. E., Kunkel, D. D., Baskin, D. G. & Schwartzkroin, P. A. Inhibition in kainate-lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J. Neurosci. 8 , 1991–2002 (1988).
Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14, 375–403 (1985).
Geiger, J. R., Lübke, J., Roth, A., Frotscher, M. & Jonas, P. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18, 1009–1023 (1997).
Ali, A. B., Deuchars, J., Pawelzik, H. & Thomson, A. M. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J. Physiol. (Lond.) 507, 201–217 (1998).
Sik, A., Tamamaki, N. & Freund, T. F. Complete axon arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin-containing interneurons. Eur. J. Neurosci. 5, 1719– 1728 (1993).
Häusser, M. & Clark, B. A. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration . Neuron 19, 665–678 (1997).
Cobb, S. R., Buhl, E. H., Halasy, K., Paulsen, O. & Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78 (1995).
Wyllie, D. J. A., Manabe, T. & Nicoll, R. A. A rise in postsynaptic Ca2+ potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron 12, 127– 138 (1994).
Bekkers, J. M. & Stevens, C. F. The nature of quantal transmission at central excitatory synapses. Adv. Second Messenger Phosphoprotein Res. 29, 261– 273 (1994).
Acknowledgements
We thank members of the Nicoll-Malenka lab for discussions, D. Selig for providing on-line data acquisition software and H. Czerwonka for secretarial support. M.F. is supported by an NIH postdoctoral training grant in Neuroscience (T32NS07067). R.A.N. is a member of the Keck Center for Integrative Neuroscience and the Silvio Conte Center for Neuroscience Research. R.C.M. is a member of the Center for Neurobiology and Psychiatry, and the Center for the Neurobiology of Addiction. R.A.N. is supported by grants from the NIH. R.C.M. is supported by grants from the NIH, HFSP, and an Investigator Award from the McKnight Endowment Fund for Neuroscience.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frerking, M., Malenka, R. & Nicoll, R. Synaptic activation of kainate receptors on hippocampal interneurons . Nat Neurosci 1, 479–486 (1998). https://doi.org/10.1038/2194
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/2194
This article is cited by
-
Development of Cortical Pyramidal Cell and Interneuronal Dendrites: a Role for Kainate Receptor Subunits and NETO1
Molecular Neurobiology (2019)
-
NETO1 Regulates Postsynaptic Kainate Receptors in CA3 Interneurons During Circuit Maturation
Molecular Neurobiology (2019)
-
The Role of Kainate Receptors in the Pathophysiology of Hypoxia-Induced Seizures in the Neonatal Mouse
Scientific Reports (2018)
-
Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function
Nature Reviews Neuroscience (2012)
-
Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1
Nature Neuroscience (2011)